Article • Promising, but in need of further validation
Implementation challenges of blood biomarkers for Alzheimer’s disease
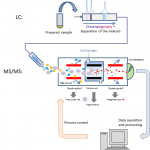
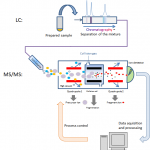
Blood-based biomarker (BBB) tests may represent the best weapon to combat the soaring rates of Alzheimer’s disease (AD) throughout the world. Existing clinically validated tests are currently deployed to facilitate diagnosis, to monitor disease and effectiveness of treatments, to quantify progression, and to determine if a patient is appropriate for treatment or participation in a clinical…