News • Combining stem cell therapy with surgery
Spina bifida: in-utero stem cell treatment approach shows promise
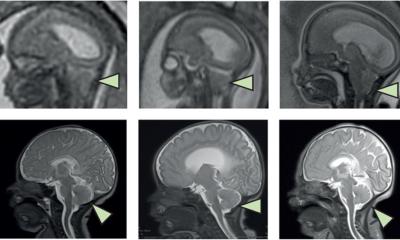
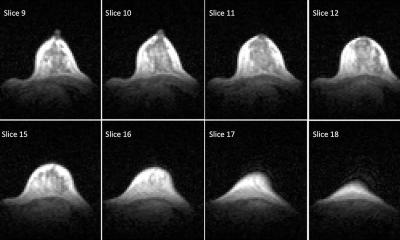
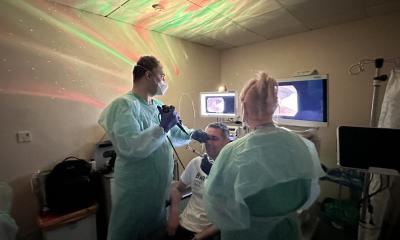
Combining stem cell therapy with standard fetal surgery before birth is a safe and promising approach to treat myelomeningocele, a severe form of spina bifida, a new study has shown.