Source: Siemens Healthineers
News • Coronavirus assay receives CE Mark
Quantitative Covid-19 test to measure neutralizing antibodies
Siemens Healthineers announced its SARS-CoV-2 IgG Antibody Test (sCOVG) has proven to measure neutralizing antibodies and has achieved CE Mark. The test is an enhanced version of the assay which became available globally this summer.
It demonstrates the ability to detect neutralizing antibodies and reports quantitative results measuring the amount of neutralizing antibodies present in a patient's blood sample. The company has submitted an application to FDA under Emergency Use Authorization.
We targeted the spike protein for our antibody tests, anticipating antibodies to this protein would eventually prove to be neutralizing
Deepak Nath
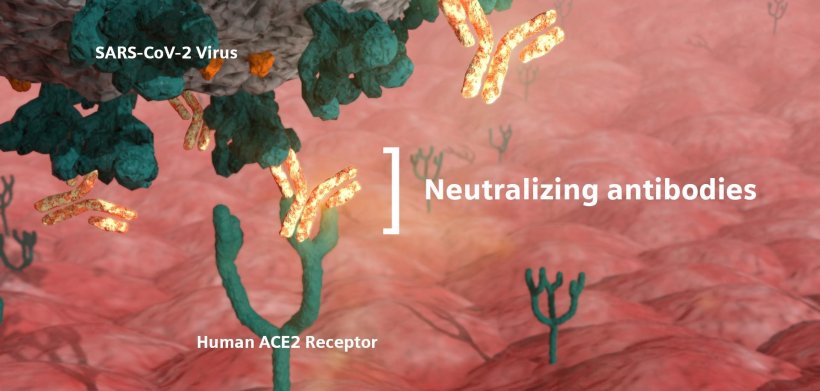
Neutralizing antibodies are critical in the fight against Covid-19 because they defend cells from infection by the virus. A virus typically produces an immune response of many antibodies that act as an army to help fight off the virus; however, only a small subset of those antibodies are capable of neutralization—blocking the virus from infecting additional cells. Those neutralizing antibodies develop either in response to natural infection or to vaccination, then subsequently bind to the virus and block infection. To measure a vaccine’s effectiveness, it is critical to identify both the presence of these neutralizing antibodies as well as quantitatively assess the likely level needed to protect against future encounters with the virus. The antibody test will make learning this information possible as vaccines are rolled out and exposure to Covid-19 is tested against immunization and the level of immunity a vaccine provides.
"At the onset of the pandemic, the scientific community had to learn about Covid-19 and how our immune systems would respond. We targeted the spike protein for our antibody tests, anticipating antibodies to this protein would eventually prove to be neutralizing," said Deepak Nath, PhD, President of Laboratory Diagnostics at Siemens Healthineers. "Adequate data is available now to confirm the spike protein antibodies are indeed neutralizing, especially those against the spike receptor binding domain. Healthcare providers can feel confident that our test will help them determine whether a patient's immune system is producing the right antibodies to stop or prevent Covid-19 infection."
The Siemens Healthineers SARS-CoV-2 IgG antibody tests are available on one of the largest installed bases of automated immunoassay analyzers worldwide, and the largest in the U.S. This includes the Atellica® Solution and ADVIA Centaur XP and XPT families of analyzers and the Dimension Vista and Dimension EXL systems.
Siemens Healthineers has distinguished itself as a provider of quality assays to aid the Covid-19 pandemic. In addition to antibody, antigen, and molecular SARS-CoV-2 tests, Siemens Healthineers offers a broad diagnostics portfolio to aid in the prognosis, treatment and follow-up of Covid-19 patients. The company’s broad and differentiated menu includes hematology, coagulation, cardiac, respiratory, inflammation and infectious disease panels. Blood gas and imaging solutions from Siemens Healthineers deliver actionable results that aid clinicians in caring for COVID-19 patients.
Source: Siemens Healthineers
19.11.2020