Article • Lab advances against doping
A powerful tool for sports drug testing
Due to the scientific and technical developments in recombinant DNA technology and protein engineering since the early 1980s, therapeutic proteins have emerged as one of the most important classes of new pharmaceuticals.
Report: Katja Walpurgis & Mario Thevis

Currently, more than 200 protein and peptide based drugs have gained approval by the USA’s Food and Drug Administration (FDA) and many more are under preclinical or clinical investigation.
These comprise recombinant versions of natural proteins (e.g. growth factors, enzymes, anticoagulants) as well as engineered proteins, protein conjugates, Fc fusion proteins, and recombinant therapeutic antibodies. Both protein re-design and chemical modifications such as glycosylation and PEGylation are strategies that can be employed to extend the serum half-life and improve the effector functions of a protein drug. Moreover, the construction of Fc fusion proteins through the attachment of an immunoglobulin G1 (IgG1) Fc domain to a protein or peptide can provide IgG-like properties, such as a long serum half-life, due to recycling through the neonatal Fc receptor (FcRn) and a slower renal clearance. Therapeutic antibodies are comparably stable molecules, which bind their targets with high affinity and specificity. Therefore, they constitute the largest and fastest growing class of protein drugs. At present, more than 50 chimeric (approx. 70% human sequences), humanised (85-90% human sequences), and fully human antibodies are approved for the treatment of autoimmune diseases, cancer, and other disorders.
Both the approved and investigational protein/peptide therapeutics comprise several drug candidates with potential performance-enhancing properties, whose misuse in sports is restricted under the terms of the World Anti-Doping Code (WADC). While protein drugs such as erythropoietin and its derivatives, human growth hormone (hGH), and human chorionic gonadotropin (hCG) can be detected by using immunological approaches such as Western blotting and luminescent immunoassays (LIAs), liquid chromatography and high-resolution mass spectrometry (LC-HRMS) are routinely used to test doping control samples for the presence of peptidic compounds such as insulins and growth hormone releasing peptides (GHRPs).
The proactive development of specific and sensitive detection methods for emerging protein/peptide therapeutics still missing clinical approval is the main task of preventive doping research. Many of the protein-based drugs currently undergoing clinical investigation have the same therapeutic target: TGF- cytokines such as myostatin, activin A, and GDF-11.
Recommended article

Article • Heroin, fentanyl, carfentanyl
Mass Spec detects illicit drugs
As a synthetic opioid approved for treating severe pain, fentanyl has shown clear medical benefits. However, in recent years, continuous abuse of fentanyl and its derived analogues substances has become a major public health issue – overdoses and deaths associated with illicitly-manufactured fentanyl rose dramatically.
While myostatin is considered as the key negative regulator of skeletal muscle mass, both GDF-11 and activin A were found to inhibit late-stage erythropoiesis. Additionally, activin A is involved in the regulation of bone formation and recent studies suggest that the cytokine could also play a role in muscle growth inhibition. TGF- cytokines exert their biological functions through binding to activin type II transmembrane receptors (ActRIIs). Consequently, agents specifically blocking the receptor-ligand-interaction are considered as promising therapeutics for the treatment of various diseases, including muscle wasting disorders and anaemia.
For cheating athletes, these drugs represent promising alternatives to classical doping agents such as anabolic steroids and erythropoietin, which can potentially be misused to illegally increase muscle mass and red blood cell count.
Current strategies for myostatin inhibition comprise drug candidates specifically targeting the cytokine such as anti-myostatin antibodies, peptibodies, and adnectins, follistatin-Fc fusion proteins, and agents derived from the myostatin propeptide, as well as multi-targeting approaches as, for example, anti-ActRII antibodies and ActRII-Fc fusion proteins acting as receptor competitors. Such decoy receptors were also developed to stimulate red blood cell production through neutralisation of circulating GDF-11 and activin A.
As some of these unapproved compounds are already available for research purposes and distributed on the black market, specific and sensitive detection methods were proactively developed, either in collaboration with the pharmaceutical industry or by using reference proteins intended for research purposes for method development and characterisation.
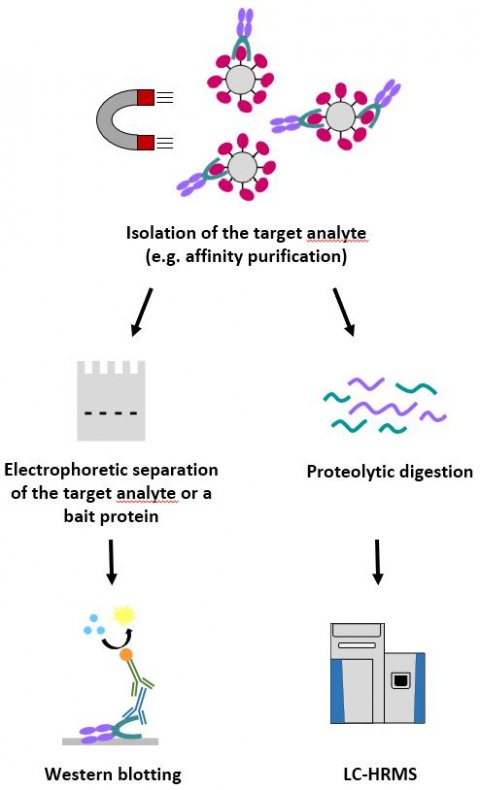
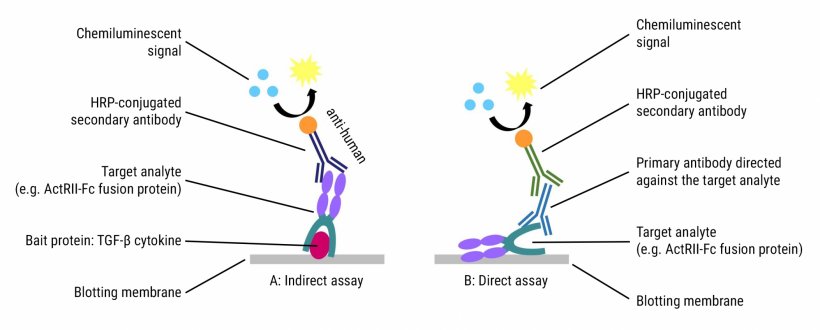
In order to detect the myostatin-neutralising antibody Domagrozumab and the anti-ActRII antibody Bimagrumab in doping control serum samples, affinity purification employing magnetic beads coupled to GDF-11 and ActRIIB-Fc was used, in combination with SDS-PAGE and Western blotting or tryptic digestion and LC-HRMS (figure 1). While the Western blot-based assay indirectly demonstrates the presence of a myostatin-binding protein with a human Fc-domain (figure 2A), the LC-HRMS methods specifically identify Domagrozumab and Bimagrumab at the amino acid level. Both the LC-HRMS assays and Western blotting approach have detection limits in the lower nanogram per millilitre range (Bimagrumab: 20 ng/mL; Domagrozumab: 50 ng/mL; sample volume: 200 µL).
Clinical study with Bimagrumab
The analysis of authentic post-administration samples obtained from a clinical study with Bimagrumab demonstrated that such a sensitivity is sufficient to detect these drugs for at least several weeks following injection.
A broad range of doping control detection methods was also developed to identify the erythropoiesis-stimulating Fc fusion proteins Sotatercept (ActRIIA-Fc) and Luspatercept (modified ActRIIB-Fc) from plasma, serum, and dried blood spots. For the isolation of the target analyte(s) from the biological matrix, magnetic beads coated with the TGF--cytokine activin A, the bacterial IgG-binding protein Protein G, or specific antibodies directed against the extracellular receptor domain were employed. The resulting sample extracts were subsequently analysed by using Western blotting or LC-HRMS: An electrophoretic separation by means of SAR/SDS-PAGE or isoelectric focusing (IEF) was combined with Western blotting in order to identify the separated intact molecules with antibodies directed against the extracellular receptor domains (figure 2B).
By contrast, the MS-based detection methods are highly specific for proteolytic signature peptides of the fusion proteins. The detection limits of the assays developed for plasma and serum varied between 0.1 and 50 ng/mL (sample volumes: 50-1000 µL). In dried blood spots, Sotatercept could be detected at concentrations as low as 250 ng/mL (sample volume: 20 µL). Again, this should enable detection windows of at least several weeks, as both Sotatercept and Luspatercept have to be administered at high doses of several hundred micrograms per kilogram bodyweight.
Most of these approaches can easily be modified to include further protein drugs as soon as authentic reference material is accessible. Moreover, they serve as proof-of-concept for the detectability of emerging TGF- inhibitors and expand the range of available tests for protein therapeutics with performance-enhancing properties.
Profiles:
Dr Katja Walpurgis studied Biology at the University of Bonn. Since gaining her PhD in 2013, she works as a postdoctoral researcher at the Centre for Preventive Doping Research/Institute of Biochemistry of the German Sport University Cologne. Her work focuses on the development of novel detection methods for performance-enhancing protein drugs by using different proteomics techniques, such as gel electrophoresis, western blotting, and high resolution accurate mass LC-MS.
Dr Mario Thevis graduated in organic chemistry and sports sciences in 1998. He gained his PhD in Biochemistry in 2001 and did post-doctoral research at the Department of Chemistry and Biochemistry at the University of California Los Angeles (UCLA) in 2002. After being a senior researcher (2003-2005) he became Professor for Preventive Doping Research at the German Sport University Cologne in 2006. In August 2017, he accepted the position of Director of the Institute of Biochemistry of the German Sport University in Cologne.
01.01.2020