Article • Mass spectrometry in patient care
LC-MS/MS: Why qualitatively high-value analysis is cheaper in the end
In the past, we repeatedly focused our attention on developments in the clinical application of mass spectrometry-based methods in patient care. Various aspects became significant.
Report: Walter Depner
Today, the use of Liquid Chromatography Triple Quad Mass Spectrometry (LC-MS/MS) for therapeutic drug monitoring (TDM) can be considered today’s standard, although classically applied immunoassays continue to be practical. However, the limitations of these techniques, e.g. non-specificity and matrix influences have immediate impact on therapy decisions. Misjudgements have fatal consequences. This raises the question whether this can still be tolerated today.

In combination with steroid analysis, the inadequacies of ligand assays due to cross reactivities among many structure homologies have been known for a long time. That is why in many areas mass spectrometry based procedures have been available for the broad routines for years or have replaced ligand assays.
For historical reasons, clinical physicians think frequently in single parameters. The exceptions are haematological diagnostics (haemograms), electrophoresis or lymphocyte differentiation, where parameter profiles are used for interpretation. With the mass spectrometric procedures, parameter profiles can be generated without a great deal of additional effort, already used for example, in steroid analysis and metabolomics. Here more rethinking is necessary. Professor Uta Ceglarek from Leipzig has already pointed to the advantages, namely smaller sample volumes, simultaneous analysis, and the absence of cross reactivities.
Dr Kromidas emphasised the progress with REIMS (Rapid Evaporative Ionisation-MS) in cancer surgery, thus the surgeon learns something about whether the tissue is benign or malignant in real time.
Technical aspects
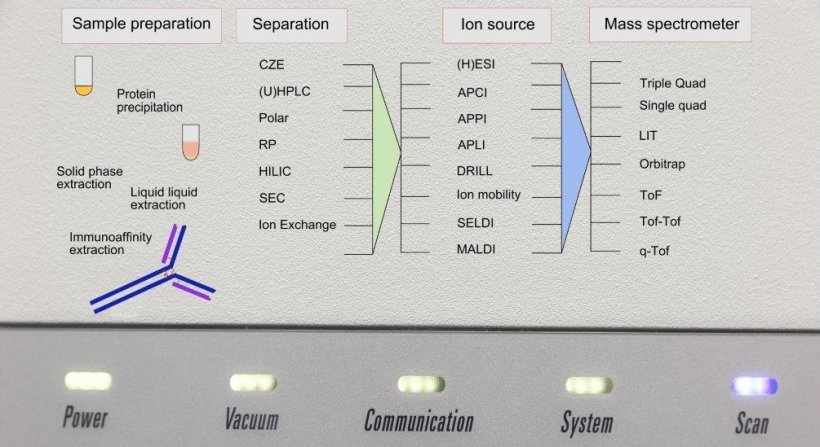
Meanwhile, the sensitivity and robustness of current LC-Tandem-MS systems has reached a degree that allows robust measurement of many medically relevant parameters, e.g. biogenic amines, steroids, vitamins and metabolites. The restriction lies in the lack of fully automated random access systems, such as those familiar for clinical chemical or ligand assay based analysers. Even if the external laboratory is less affected than the hospital laboratory, there are also various approaches to closing this gap.
One way is the special ‘front ends’ meanwhile available, compatible with the LC/ MS coupling; open systems that permit in-house development sample by sample rather than the batch processing. In the case of hospital patient care some parameters are desired potentially 24/7, e.g. TMD, which can be accomplished with such systems. Also, the first fully automated systems have been launched in the market. However, as closed systems they only provide procedures that the manufacturer considers interesting and offers. Together with available KIT methods, users lacking methodical experience also have the opportunity to apply MS based procedures.
Dr Thomas Stimpfl, from Vienna, gave special attention to aspects such as sample preparation and LIMS connectivity. These features are normal in commercial, fully integrated analysers. Increasingly they are introduced even with In-house systems and contribute substantial error reduction and time saving compared to the manual method. The advantage of a fully integrated system is that the manufacturers can match the individual components optimally, so that space-saving systems are also possible in the random access modus. Such developments are also conceivable in-house. As yet it is impossible to anticipate the down times and MTBFs with fully integrated systems.
Advances in the development of ion sources, e.g. DRILL technology lead to 10-fold increases in sensitivity. Coupling with ion mobility separation is suitable for suppression of isobar interference and thus delivers improved specificity. The application of immune-affinity purification in the context of sample preparation for LCMS ought to permit an up to 1000-fold enhancement of the peptide target. That could mean the use of a stable isotope marked internal standard to improve reproducibility. Even the determination of enzyme activity, by measuring the substrate, can be done sensibly using LC-MS/MS. In the laboratory of Prof Brian Kevil in Manchester the renin activity is determined routinely with LC-MS/MS.
The wide range of high-resolution mass spectrometers permits use for database searches and identity confirmation of analytes as well as separation from interferences, whereby compromises in sensitivity are needed here.
Current and future medical aspects
The work of the last decade has proved that mass spectrometric methods are not only qualitatively better compared to ligand assays but also can become routine
Our own data indicates, for example, that testosterone in women with an automated immunoassay deviates within a range from -80% to +80% from the LC-MS/MS measurement, whereby the overall error for LC-MS results is merely a few per cent. The data situation is similar for determining vitamin D with the LC-MS/MS in comparison to the immunoassay. The practical side in the Wisplinghoff lab and in our own shows that the LC/MS can be used well for large series, such as vitamin D and steroids. It has great potential for improving analytical quality compared to the commercial ligand assay.
In the context of therapy control for multiple myeloma, the use of LC-MS/MS permits specific detection of myeloma proteins with a 2000-times higher sensitivity than the immune fixation electrophoresis currently used. That means bone marrow follow-up could become superfluous and earlier recognition of minimal residual disease, respectively a relapse, would be possible. Assays for tumour markers are known as difficult to compare with each other. Hence the evidence obtained from this parameter is only of limited utility. Potentially there are new aspects resulting from more specific determination of these antigens using LC-Tandem-MS.
The work of the last decade has proved that mass spectrometric methods are not only qualitatively better compared to ligand assays but also can become routine. Nevertheless, these new methods have not entered daily work everywhere. Even in the context of research studies inferior methods are often still used. Reasons for this could be that test development plays almost no role in the professional training for laboratory medicine. Frequently the medical side also lacks the methodological understanding. However the questions arise in laboratory medicine.
It is the specialists’ responsibility in laboratory medicine to assure that analysis remains up to date. This expertise can only come from him since the exclusively clinical physician can only roughly estimate the influence of the methods used on the diagnostic process and scientists working only methodologically lack medical expertise. The broader development of in-house procedures and the related expertise also makes it possible to develop scientific questions in the out-patient area, a field currently completely neglected, but which affects a large part of the population.
Economic aspects
Practical aspects such as employee qualification and technical skills for operating the machines are evaluated differently. Our experience is that reliable routine use of LCMS is possible after appropriate initial training. The necessary knowledge for methodological development grows naturally and constitutes a laboratory’s valuable capital.
With commercial fully automated systems it is important to recognise that here the manufacturer’s income aspects are drivers. Parameters are offered from which the manufacturer expects the greatest profit by virtue of high throughput and not necessarily those that are the most sensible medically. Naturally, commercial fully-integrated systems will always be more expensive than the corresponding in-house procedures. This places the laboratory in the (contradictory) situation that the income for the high volume and hence cost-effective parameters flows to the manufacturer, while the more expensive special parameters must also be performed at greater expense with an in-house method with a low number of samples. However, the general use of in-house tests results in a reasonable mixed calculation with synergies and broader utilisation of available expertise. This factor appears especially important given current economic pressure with everyone’s earnings expectations.
As a rule, although the investment costs for LC-MS/MS are substantial, subsequent costs for test development and operation are low. 700 euros for a HPLC column may seem an expensive acquisition, but with a service life of 20,000 injections yields a price of 0.035 euros per measurement. Naturally, the establishment of expertise is also not free, yet it is a sustainable investment. In today’s competitive situation this gives the lab the opportunity to react flexibly to changing demands and still offer the highest level of analysis.
Quintessence
Funding entities must recognise that qualitatively high-value analysis constitutes a less expensive alternative to merely cheap test methods
The application of LC-MS/MS in clinical routines is still viewed as an expensive niche method for highly specialised analysis. This perception is obsolete since there are many robust and reliable options available to use. It is desirable for laboratory medicine, as a specialty, to focus more on the knowledge in methodological development, also for more demanding procedures, such as mass spectrometry, currently performed so often by chemists. The methodological work would open many more diagnostic options in the clinical context than are currently practised. Funding entities must recognise that qualitatively high-value analysis constitutes a less expensive alternative to merely cheap test methods. This can gain importance when medico-legal implications are considered.
Profile:
Professor Uta Ceglarek is Assistant Director at the Institute of Laboratory Medicine, Clinical Chemistry and Molecular Diagnostics (ILM) at University Hospital Leipzig, where she has headed the newborn screening laboratory since 2005. After gaining her degree in chemistry and doctorate in analytical chemistry, Ceglarek specialised in toxicology and clinical chemistry. In 2010 she wrote her habilitation on clinical metabolome research. From 2000 she has been working to develop mass spectrometry diagnosis for newborn screening, therapeutic drug monitoring and metabolism. She is a member of the board at the German Society for Newborn Screening (DGNS) and a spokesperson for the Clinical Mass Spectrometry section at the German Society of Clinical Chemistry and Laboratory Medicine (DGKL).
25.04.2019