Laboratory automation
On the leading edge of automation for medical laboratory testing, Siemens Healthcare rolled out an impressive suite of new products at IFCC WorldLab 2014 in Istanbul, Turkey that are ‘exactly aligned, and even anticipate, customer needs in central lab operations,’ according to Franz Walt, CEO for the Chemistry, Immunoassay, Automation and Diagnostics IT business unit within Siemens’ Diagnostics Division.


Managers for centralised medical laboratories are under a lot of pressure today, he said. Under-staffing and a shortage of available candidates for open positions is one of the most frequent concerns he hears speaking with customers.
The demands on lab technicians is more demanding than ever while economic pressures are also putting the squeeze on managers’ ability to optimally run their operations as they are repeatedly asked to do more with less funding and fewer people.
According to Walt, ‘these are the forces that are driving labs toward automation. You cannot substitute with a machine the need for people to be part of the process, but we are helping labs cope with these pressures by doing more with fewer people.’
‘With these new products we have taken the concerns of customers very seriously to improve functionality and to give an overall workflow optimisation. This is the first line of instruments truly built for automation and they are ready to go without extra robotics needed to connect them,’ said Walt.
‘We are making a significant contribution to increasing productivity and to assuring very predictable turn-around times for lab tests. Also, by minimising the need for manual steps, we reduce the opportunity for error,’ he said.
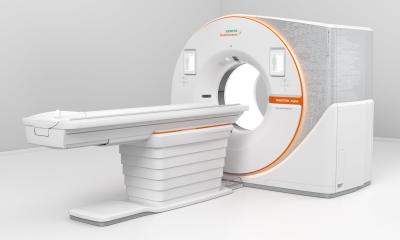
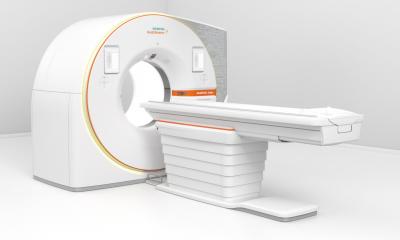
New products added to the company’s portfolio include the ADVIA Chemistry XPT System and ADVIA Centaur XPT Immunoassay System, designed to enable continuous operations, facilitate delivery of timely, accurate results, and ease training. Also presented in Istanbul were Siemens’ new analysers for the Laboratory Haematology sector, the Sysmex CS-5100 Haemostasis System and ADVIA 2120i Haematology System.
These front-line systems connect with the enhanced data flow capabilities in Siemens’ CentraLink Data Management System and Aptio Automation, key components for integrating an advanced track-based automation system. At the IFCC congress, the new Tube Inspection Module was linked with Aptio Automation to demonstrate how labs can reduce pre-analytical errors and minimise the risk for tube mismatch. The key word that pulls together the portfolio is multi-disciplinary, said CEO Walt.
‘To this point our efforts have been in meeting requirements for the main line applications in routine testing. With these new products we have moved with our customers to the next level of more comprehensive, multi-disciplinary testing,’ he explained.
New instruments for haemostasis and haematology complete the portfolio for analysis, while expanded capabilities of software and middleware enables the multi-disciplined management of the total workflow for central lab operations.
‘Lab analysis is a complex constellation where instrument automation is only one part of the solution. The other key element is information technology. The CentraLink system version for 2015 supports paperless workflow and embraces the multi-disciplined approach by bringing together haematology, chemistry and immunoassay analytics ,’ Walt said.
‘There is also something we call SLIM [syngo Laboratory Inventory Management] that assists automation efforts by tracking a customer’s inventory, including both Siemens’ products and third party products. It will also propose suggestions for reordering and can connect to e-commerce sources to help with that.’
‘Through all of these components operations become increasingly integrated. We not only optimise workflow but reach beyond to data automation and add connectivity to the rest of the constellation in the lab,’ said Walt.
Moving beyond the Siemens’ universe of products in the complex constellation of lab automation, the company’s new line of instruments and the middleware linking the machines to information technology conform to newly adopted standards developed through the non-profit group, Integrating the Healthcare Enterprise (IHE).
Rather than competing with each other using proprietary codes for data sharing, lab instrument manufacturers agreed to form a consortium called the IVD Industry Connectivity Consortium (IICC) and to work through IHE to develop open standards.
The IICC membership is impressive, including the top makers of clinical instruments such as Abbott Diagnostics, Beckman Coulter, Becton Dickinson, bioMérieux, Data Innovations, Orchard Software, Ortho Clinical Diagnostics, Roche Diagnostics, Siemens, and Systelab Technologies.
A key change resulting from this collaboration is an upgrade to a piece of hardware that removes what had become a bottleneck to high-speed data transfer. Up to this point, and even today on legacy instruments, vital patient information from blood work ups and molecular diagnostic assays ran through the old 9-pin cables that are now hard to find on the back of computers. (See European Hospital Issue 03, 2012)
Some of these lab analysis instruments transfer data at the extremely low-bandwidth rate of 9600 baud, requiring 15 minutes to upload one megabyte, where today most smartphones can transfer the same data in one second.
According to CEO Walt, the Siemens’ CentraLink Data Management is a system capable of connectivity to multiple lab instruments and is flexible to accommodate a range of connectivity requirements.
‘This kind of optimisation is the name of the game today, and Siemens is absolutely leading the market,’ he said.
Profile:
Franz Walt brings 25 years of experience in healthcare to his responsibilities as head of the Chemistry Automation Immunoassay business unit for Siemens Healthcare Diagnostics. His career has covered assignments in pharmaceuticals, diagnostics, and medical devices across a mix of customer groups in Asia Pacific, Iberia, Latin America, Europe and North America.
29.07.2014