Sponsored • Cardiac troponin I concentration measured at POC
Triage aided by a quick sensitive test
Large proportions of patients can be safely triaged either to rule out discharge or rule in lifesaving management – if following the European Society of Cardiology (ESC) Guidelines Class I recommendation of two serial measurements of hs-cTnI on admission and after one hour, if there are assay specific cut off values for the 0/1 algorithms.
The Pathfast hs-cTNI assay is an approved system to determine highly sensitive troponin I recommended in the ESC Guidelines 2020, within the quick 0/1 hour rule out diagnostic algorithm. The manufacturer, LSI Medience Corporation, reports:
"Pathfast hs-cTnI is a sensitive Chemiluminescent Enzyme Immunoassay (CLEIA) for the quantitative measurement of cardiac troponin I (cTnI) concentration in whole blood or plasma at the point-of-care (POC). The Pathfast assays are designed for Near Patient Testing (NPT) and can be used as an aid in the diagnosis of acute coronary syndromes (ACS) and in the risk stratification of patients presenting with suspected acute coronary syndromes such as chest pain in a hospital. Reagents are single use in all-in-one cartridges and up to six tests in parallel can be tested in one run. Besides highly sensitive troponin I, additional biomarkers as NT-pro BNP, D-Dimer, Myoglobin, CK-MB mass, hs-CRP and the new innovated emergency sepsis marker Presepsin can be measured at the same time with superior quality.
In less than 17 minutes the Pathfast system provides highly accurate, precise test results out of whole blood, plasma and serum similar to central laboratory analyser.
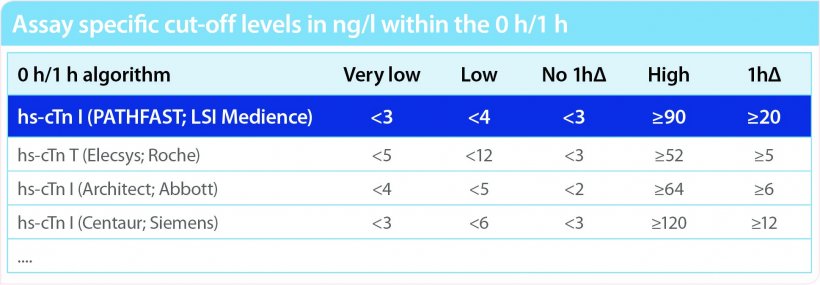
In clinical studies, Pathfast hs-cTnI assay has been evaluated for a 99th percentile upper reference limit of 29.0 ng/L at an imprecision of 6.1%, which fits the criteria of hs-cTnI assays recommended by IFCC and ESC. Moreover, gender specific cut off values were established and a 0/1 hour Rule-out and Rule-in algorithms of NSTEMI patients were evaluated. Recommended by the 2015 and 2020 ESC guidelines cTnI concentration were measured from 1,221 patients with suspicion of NSTEMI (669 for derivation and 610 for validation) using the Pathfast hs-cTnI assay in EDTA plasma samples obtained at 0 hour and one hour after admission of patients to the chest pain unit (CPU). As presented in recent publication, the identified cut offs for 0 hour rule-out showed 100% and for 0/1 hour rule out 99.7% negative predictive value (NPV). For 0/1hour rule-in 80.1% positive predictive value (PPV) were shown. In total, more than 62% patients could be triaged successfully in this clinical study."
16.11.2020