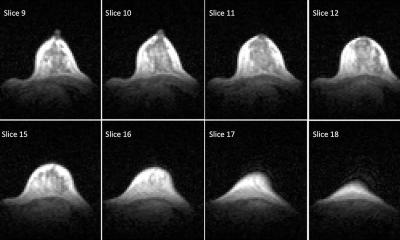
News • LLMs confirm delusions
Mental illness and AI chatbots: a dangerous match
Worsened delusions, mania, suicidal ideation, eating disorder: People with mental illness who use AI chatbots risk experiencing a worsening of their condition, a new study shows.