News • Mammography
Hologic and Tromp Medical to provide systems for Dutch Breast Cancer Screening
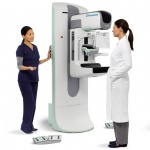
Hologic, Inc. announced that it will provide all mammography systems for the Dutch Breast Cancer Screening Program in partnership with Tromp Medical, Hologic’s distributor in the Netherlands. Under the tender, Hologic’s new, state-of-the-art 3Dimensions mammography systems will be installed in mobile and stationary screening facilities across the country, starting in 2018. The multi-year…