Article • Interpreter of maladies
Will AI help realize the promise of precision medicine?
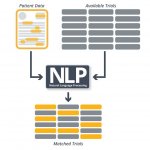
Machine learning and artificial intelligence (AI) have the potential to transform cancer treatment management worldwide. Their ability to rapidly analyse and integrate routinely acquired diverse data will improve the accuracy and effectiveness of precision medical treatments.