News • Microfluidics and electrochemical transduction
Paper-based device for rapid diagnosis of lung diseases
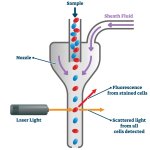
A new device that combines microfluidics on paper, electrochemical transduction and immunoassays on magnetic nanoparticles is useful for easy and rapid diagnosis of lung diseases.