© Clayton Metz, Virginia Tech
Article • Tissue-engineered glioblastoma model
3D brain tumour in a dish to personalize cancer treatment
It is the size of a common pencil eraser, but it could have a huge impact on the therapy of glioblastoma: Scientists in Virginia have developed a novel 3D tissue-engineered model of the brain tumour microenvironment, which can be used to assess how the glioma cell invades healthy tissue, proliferates, and reacts to chemotherapy drugs.
Report: Cynthia E Keen
Glioblastoma, the most common and most malignant form of brain cancer, is notoriously difficult to treat, due in part to its invasion into surrounding tissue where the tumour cells become more resistant to therapy. Median patient survival is five months.
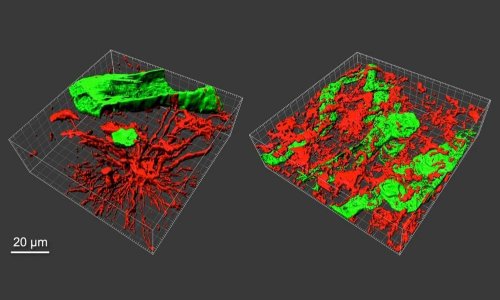
Tumour microenvironments (TMEs) – the tissue that surrounds a tumour – are known to contribute to cancer progression. The brain TME contains cells specific to the central nervous system, including astrocytes and microglia. Understanding how TME functions and how glioblastoma tumour cells grow, and being able to control, manipulate, and tune its elements, may help enable the development of more effective, individualized therapies for glioblastoma patients.

© Matt Chittum, Virginia Tech
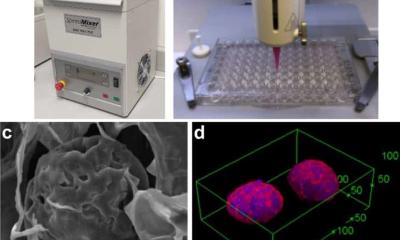
The 3D model developed by Jennifer M. Munson, PhD, an associate professor of biomedical engineering at the Fralin Biomedical Research Institute of Virginia Tech in Roanoke, is an accurate recreation of TMEs: It contains cells unique to the central nervous system, in ratios based on those found in patients, as well as glioblastoma stem cells and interstitial fluid flow, the movement of fluid between and around cells in tissues. Dr Munson and a multi-institutional team of researchers describe the model’s detailed composition and what they learned from it in an article published in NPJ Precision Oncology. ‘Our goal is ultimately to develop a personalized medicine approach in which we can take a patient’s tumour, build a model of that tumour in a dish, test drugs on it, and tell a clinician which therapy will work best to treat it,’ said Munson.
Inter-patient differences have huge impact
The research team examined individual and synergistic effects of the cellular and biophysical glioblastoma microenvironment on patient-derived glioma stem cell outcomes, bidirectional intercellular communication between glioma and glial cells, and the effectiveness of various chemotherapy drugs on destruction of tumour cells. They determined that:
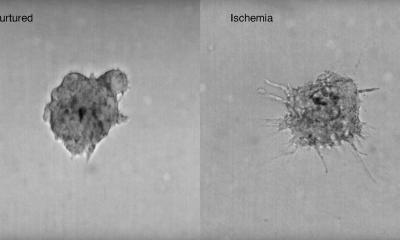
- Invasive regions primarily contain neural astrocytes and microglia;
- Glioma cell invasion is patient specific and depends on the TME context. Inter-patient differences are the greatest contributor to invasion. The addition of glial cells or a combination of glia and interstitial fluid flow can meaningfully influence glioblastoma stem cell invasion either positively or negatively;
- The proliferation of tumour cells is highly dependent upon a patient’s glioblastoma stem cell line(s) and how a specific cell line interacts with fluid flow;
- CCL2, a chemokine which guides a variety of immune/inflammatory cells to the site of a tumour, plays a role in TME-driven enhancements to glioma malignancy and may impact individualized cancer responses;
- Therapeutic drug response exhibited in the model could partially predict responses seen in in-vitro tests on laboratory mice. Further testing is required to expand on these findings and incorporate other important factors, such as the blood-brain barrier (BBB), which impacts drug transport and provides important pro-tumour signalling.
‘Results of our testing varied widely,’ Munson said. ‘This highlights the importance of a personalized medicine approach to glioblastoma and the value of being able to recreate an individual patient’s tumour microenvironment.’
The team is working to obtain more patient data and establish more academic-clinical collaborations to better identify which model components and metrics are needed to establish transformative predictive power in glioma therapy. Munson told European Hospital that the team is also leveraging the tunability and ease of implementation of this system to use it to identify new therapeutic targets and to understand the complex interactions between the cells in the brain and tumour cells in disease.
Profile:
Jennifer M. Munson, PhD, is an associate professor in tissue engineering in the Biomedical Engineering and Mechanics Department of the Virginia Tech College of Engineering. She is director of the Munson Lab at VT’s Fralin Biomedical Research Institute, and a co-director of the Virginia Tech Cancer Research Alliance. Munson began to develop 3D tissue-engineered models for cancer research starting in 2014. She collaborates with engineers and biologists throughout the world.
16.11.2022