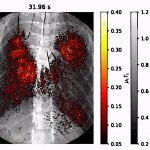
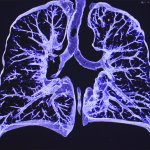
News • Improved accuracy and efficiency
AI could improve CT screening for COVID-19
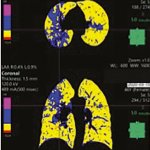
Researchers at the University of Notre Dame are developing a new technique using artificial intelligence (AI) that would improve CT screening to more quickly identify patients with the coronavirus. The new technique will reduce the burden on the radiologists tasked with screening each image. Testing challenges have led to an influx of patients hospitalized with COVID-19 requiring CT scans which…