News • Aspergillosis monitoring
Chip-based model to observe fungal spread in the lung
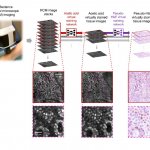
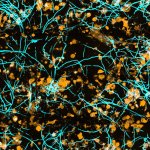
A chip-based infection model developed by Jena researchers enables live microscopic observation of damage to lung tissue caused by the invasive fungal infection aspergillosis.