News • Venous cannula system
New circulation implant to bridge the waiting time for donor heart
With the first-in-man implantation of the Berlin Heart Venous Cannula at the LMU University Hospital Munich, Germany, Berlin Heart offers patients with a failing Fontan circulation a unique chance to survive the waiting time for a donor heart.
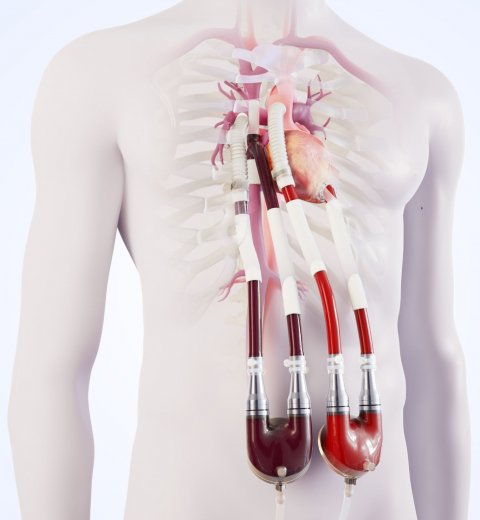
© Berlin Heart GmbH
These patients are in a life-threatening condition: their health has deteriorated so much that they desperately need a new heart, but because of their poor end-organ function, amongst other issues, their chances to survive on the waiting list are low. The therapy was developed to improve the end-organ function and hemodynamics of these patients with univentricular physiology through the support of the sub-pulmonary circulation. Thus, the Berlin Heart Venous Cannula builds the bridge to transplant for patients with failing Fontan circulation.
Improvements of surgical therapies and patient care ensure that even children with complex congenital heart defects, such as a single ventricle, may survive and live into adulthood. Nevertheless, for many patients, the time comes, when due to worsening heart failure the only remaining hope is a donor heart. With the aim of giving these patients a chance to bridge the sometimes very long waiting times, Berlin Heart has developed the Excor Venous Cannula. Based on an idea for a specific venous cannula replacing the right atrium and modified according to the original design input of Dr. Eugen Sandica, Pediatric and Congenital Heart Surgeon from the Heart and Diabetes Centre Bad Oeynhausen, this cannula was specifically developed for the unique challenges of a failing Fontan circulation. The cannula is designed to reduce the complexity of surgery, improve the sub-pulmonary hemodynamics and enable the end-organ recovery.
Artificial sub-pulmonary support is the only way to normalize hemodynamics, allow for organ recovery during mechanical support therapy and consequently has the potential to lower mortality and morbidity on the waiting list
Nikolaus Haas
The Pediatric Heart-Team from the LMU University Hospital Munich was the first to use this device. The specialized pediatric cardiac surgical team led by Prof. Juergen Hoerer (Pediatric Heart Surgeon) implanted this cannula. Prof. Nikolaus Haas (Pediatric Cardiologist and Intensivist) emphasises the valuable new option: “These patients often have adequate function of their ventricle but extremely poor circulation due to this unphysiological flow pattern after Fontan surgery. In this situation, artificial sub-pulmonary support is the only way to normalize hemodynamics, allow for organ recovery during mechanical support therapy and consequently has the potential to lower mortality and morbidity on the waiting list. Moreover, we are confident that we can reduce the risk of the heart transplantation in failing Fontan hemodynamics.”
Patients with Fontan circulation suffer from many associated end-organ dysfunctions caused by poor blood circulation like liver failure (Fontan-associated-liver-disease (FALD)), severe problems of the gut with uncontrollable diarrhoea (protein losing enteropathy) or life threatening lung affections (plastic bronchitis). To overcome this, they urgently need a donor heart. While these significant comorbidities indicate a heart transplantation, at the same time, they worsen its chance of success.
“The specifically designed Excor Venous Cannula facilitates a standardized implantation technique and enables mechanical circulatory support of the sub-pulmonary circulation in patients with failing Fontan for the very first time”, says Dr. Sandica. “This cannula permits the use of the Excor VAD as sub-pulmonary support alone (RVAD) or in combination with a LVAD, in a so called BVAD mode in a majority of patients with failing Fontan circulation, thereby serving an unmet medical need.”
The clinical improvement due to this novel cannula will be assessed within a prospective, international, multi-centre registry study “RegiVe” - Registry to assess the safety and feasibility of the subpulmonary support with the novel Venous Cannula in patients with failing/absence of the right heart. This study will take place at ten experienced centres in five European countries and will collect data on clinical condition, organ function, quality of life and outcomes, with up to a one–year follow-up. Dr. Ares K. Menon, Cardiac Surgeon and General Manager of Berlin Heart GmbH says: “The Excor Venous Cannula enables our clinical partners to build a bridge to transplant for patients with failing Fontan circulation. Our ambition is to reduce mortality and morbidity on the waiting list and increase the chances of these patients for a successful heart transplantation.“
Source: Berlin Heart GmbH
12.03.2021