Interview • Diagnostics
Iron deficiency and anaemia
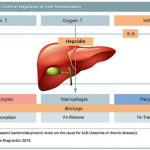
Iron deficiency and resulting anaemia cause fatal comorbidities worldwide. Despite this, they are generally underestimated. Professor Lothar Thomas, specialist in laboratory medicine at Central Laboratory of the University Hospital of Frankfurt/Main, is calling for more information about the new laboratory parameters for diagnosis and monitoring of iron deficiency and iron substitution therapy.