Infections in joint surgery: high risk for fracture patients
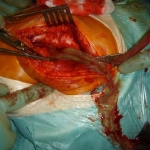
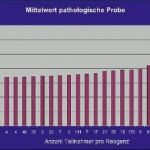
Postoperative infections after knee or hip joint replacements are among the most feared complications in orthopaedic surgery. At the EFORT Congress in London current research was presented that provides new insights in this field: Fracture patients are especially vulnerable to infection. New biomarkers should improve early diagnosis of risky infections.