Danes take pathology software worldwide
Having convinced medical labs across Denmark that its suite of image analysis software can provide a solution to the crushing burden of in vitro diagnostics (IVD), VisioPharm is offering it to pathologists worldwide
Having convinced medical labs across Denmark that its suite of image analysis software can provide a solution to the crushing burden of in vitro diagnostics (IVD), VisioPharm is offering it to pathologists worldwide
The problem is clearly visible: the increasing number of diagnostic tests for an ever-greater number of patients, but with fewer and fewer pathologists to review and report on test results. In Denmark, for example, 200 pathologists serve a population of five million. Across the bridge, Sweden also has 200 pathologists but 10 million people.
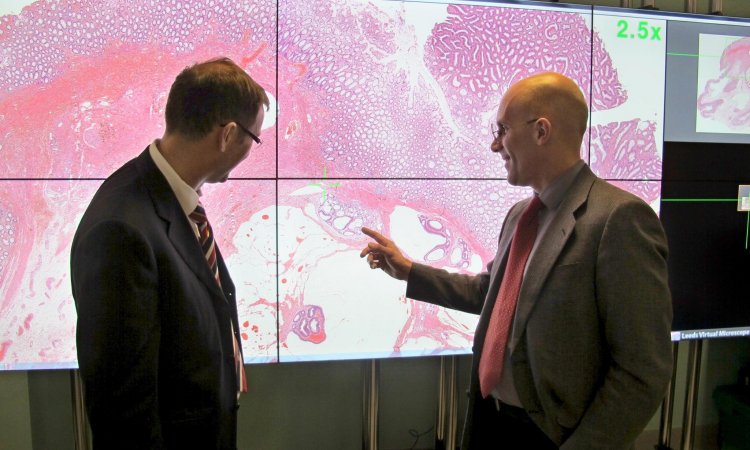
Looking inside the laboratories, the situation becomes even more complicated, according to VisioPharm’s founder and CEO, Dr Michael Grunkin. ‘There are two major difficulties for pathologists, one has to do with data quality and the other with costs and the turnaround time for any kind of IVD,’ he explained.
‘All over the world there is a consolidation of resources for pathology. Budgets are being cut constantly. Yet, there is a need to increase the capacity for patient throughput and reduce the cost per patient – all of this while maintaining, or improving, data quality.
‘Data quality is a huge problem, both for the reproducibility and the diagnostic accuracy of results of tests. One of our partners, working with 400 pathology labs worldwide, did a quantitative study showing there is a tremendous variation between pathologists, as well as variability between pathology labs. As a patient this means that if you go to two different pathology labs the treatment decision could be completely different simply because of this variability.
More tumour panels will be launched as soon as 2015
‘These are the problems we set out to solve and we have been able to improve the reproducibility of results significantly. Our software saves time, improves testing and improves reproducibility. It’s better, faster, cheaper, and we have massive amounts of clinical data to support this,’ Dr Grunkin emphasised.
Developed and refined over 12 years and featured in over 450 scientific publications, up to now,VisioPharm has deployed more than 350 of its digital pathology systems worldwide.
Thanks to advances in scanners and the processing power of computers, specimen slides can now be digitised and automatically read with the results fed into a data system for a more standardised interpretation by pathologists.
Now VisioPharm is bringing these same advantages to standardise the results of Immuno-histochemistry from IVD tests to deliver results faster and at lower cost.
‘This means we can automate certain steps in the diagnostic workflows to the point that non-pathologists may be able to perform technical aspects of testing and analysis and then pass these results to the pathologist for review. This becomes very important because it allows clinical labs to efficiently utilise their pathology resources,’ explained Dr Grunkin.
‘For our first release we’ve focused on IVD tests used for breast cancer. We will have other tumour panels as soon as next year, probably for malignant melanoma, or skin cancer, and after that for gastro-intestinal cancer and prostate cancer.”
Studies to validate time-savings for pathologists
The VisioPharm CE-IVD diagnostic image analysis software for breast cancer was granted the CE Mark of approval in 2013.
Currently the company is conducting a multi-centre evaluation study to document the quality of the data generated automatically, and also to validate the time-savings for pathologists.
Centres participating in the study are located in Sweden, Norway, Switzerland, Ireland, Germany and the United States. Preliminary results are due by the end of 2014.
24.06.2014