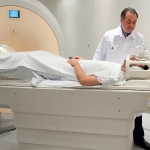
Hybrid imaging: PET/MR climbs the diagnostic ladder
Experts across Europe believe the combination is beginning to demonstrate its broad potential as a hybrid imaging tool

Experts across Europe believe the combination is beginning to demonstrate its broad potential as a hybrid imaging tool
UK researchers are working on a new MRI technique called hyperpolarised MRI – or Dynamic Nuclear Polarisation (DNP) – that can utilise more of the available nuclei than traditional MRI, helping to overcome some of its limitations by increasing sensitivity 10,000-fold or more. DNP is part of a longer-term aim to improve cancer mortality with the help of novel cancer imaging tools.

When asked about his vision of imaging in the year 2020, Professor Bernd Hamm MD, director of the three radiology clinics at the Charité University Hospital in Berlin, qualified his focus: ‘Technology is always only a vehicle. When we talk about road traffic, we don’t talk about the design of cars but about structural issues’

Virtual FDG-PET/CT bronchoscopy has been found to be a technically feasible tool for the detection of lymph node metastases in non-small cell lung cancer patients with good diagnostic accuracy, according to researchers at the Department of Diagnostic and Interventional Radiology, University Hospital Dusseldorf and Essen.

Quick, simple and comfortable – the RADbook 2012 on your iPad. Download the RADbook app free of charge today and have all state-of-the-art diagnostic imaging systems at your fingertips.

A series of papers presented at the European Congress of Radiology on Friday have highlighted how hybrid imaging is helping radiologists achieve better results in the diagnosis of patients’ conditions. In a session focussing on molecular imaging and entitled “Hybrid imaging: PET-CT and MR-PET”, findings from ten different research papers were detailed by radiologists from Italy,…
Radiology constantly evolves. There are technical advances in terms of the capabilities of various modalities, greater clarity from contrast agents that are also safer for patients, and innovation in techniques that gains even greater performance from existing equipment, or enables further development.
Royal Philips Electronics is announcing 510(k) clearance from the Food and Drug Administration (FDA) for the company’s first commercially available whole body positron emission tomography/magnetic resonance (PET/MR) imaging system, the Ingenuity TF PET/MR.
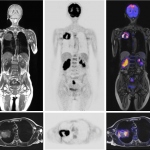
Although like a conventional MR scanner the unassuming exterior is misleading. The casing houses a powerful interior. This is the new Siemens Biograph mMR, a hybrid that contains a specially developed PET component fully protected against magnetic field interference.

In recent years, combined examination methods have increased, whereby two examination methods are used in a parallel examination, rather than performed separately. Frederik Giesel MD, Associate Professor of Radiology at the Nuclear Medicine Department, University of Heidelberg, and Philip Herold (Dipl. Econ.), Project Manager at RICT Heidelberg, report on the benefits.
Preliminary research presented at SNM’s 58th Annual Meeting is breaking new ground for the development of a brand new hybrid molecular imaging system. Simultaneous positron emission tomography (PET) and magnetic resonance imaging (MRI) is providing important diagnostic information about soft tissues and physiological functions throughout the body. Scans focused on screening suspicious lesions…
Pushing the power of scanners creates new world for imaging, but whilst high-field magnets bring new capabilities they also pose new challenges for clinicians. John Brosky reports
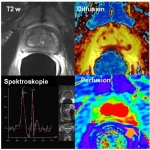
Thinking of the future of imaging, inevitably PET-MRI springs to mind. The fascination of this novel hybrid technology is great, seeing how it combines the best from three imaging areas: anatomy, function and metabolism. The further development of functional procedures in oncology is raising particularly high expectations. However, how extensive the use of this potentiated image information will…

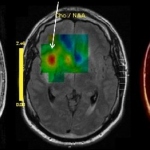
Anti-seizure medication is not successful in all patients, while in others such medication can have side effects. In recent years significant technical advances have delivered better imaging results, which, combined with growing demand for a surgical solution from patients whose medications do not control seizures, or those not wanting to take medication constantly, has led to an increase in…

Whole-body hybrid PET-MR scanners are emerging on the market and are expected to have a significant impact in diagnostic imaging particularly in oncology applications and also in other clinical domains, such as cardiology, inflammatory and infectious disease, as well as in neurological applications.
Royal Philips Electronics is announcing CE marking for the industry’s first commercially available whole body PET/MR imaging system, the Ingenuity TF PET/MR*. This new system, being launched as the first new Philips modality in ten years, integrates the molecular imaging capabilities of PET with the superior soft tissue contrast of MR to image disease cells as they proliferate in soft tissue.…
Even more precise diagnoses, even better process controls -- the future of MR-PET technology has dawned. The first commercial, full-body hybrid scanners are either waiting in the wings or already installed. But what does the introduction of the MR-PET really mean for clinical practice? Professor Heinz-Peter Schlemmer MD, Head of the Radiology Department at the German Cancer Research Centre in…

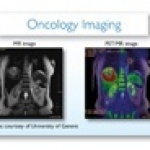
Something that often obstructs a pioneering medical spirit is simply a practicality: the lack of space. For many hospitals, investment in new medical equipment is linked with construction and reshaping the hospital’s architecture – sometimes impossible because of the infrastructure. This was precisely the situation at the University Hospital Geneva (Hôpitaux Universitaires de Genève = HUG)…
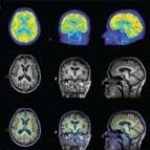
2010 workshop in Aachen, Germany, in March, were welcomed by Professor Thomas M Deserno, head of the Medical Informatics Department at RWTH Aachen University and organiser of what turned out to be an ‘outstanding’ scientific programme.
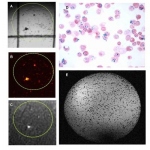
Since June 2009, the focus of research in the European Network for Cell Imaging and Tracking Expertise (ENCITE) has been on finding biomarkers to aid cell transplantation. Funded with €11 million from the European Commission (EC), this major project that runs until 2013, involves 10 countries. Their work is coordinated by the European Institute for Biomedical Imaging Research (EIBIR) network,…

The technological integration of positron emission tomography (PET) and magnetic resonance imaging (MRI) has been the dream of molecular imaging experts and engineers for some time. Now, the German Science Council has agreed to provide 6.56 million funding to install a whole-body MRI-PET prototype in the centre of excellence for imaging procedures at the radiology clinic in Eberhard-Karls…

For many indications, because PET-CT produces a very high accuracy for many tumours, this modality is the gold standard, Prof. Reiser confirmed. It also enables good observation of the course of the disease. After an injection of radioactive tracers we can visualise increased metabolic activity in great detail and with high sensitivity. This is an increasingly important issue not only in primary…

Integrated PET/MRI systems will permit the simultaneous acquisition of molecular, functional and structural parameters. The combined strengths of PET (high sensitivity and specificity, but relatively low spatial resolution) and MRI (high resolution, but low sensitivity) is the most attractive feature of multimodal imaging with hybrid scanners. Their application could substantially contribute to…
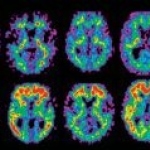
The so-called 11-labeled Pittsburgh Compound B ([11C]PiB) type of positron emission tomography (PET) may be useful in a non-invasive assessment of the formation of Alzheimer's disease-related plaques, according to a study that will appear in the October 2008 issue of Archives of Neurology.

For individualised radiotherapy, high-precision delineation and characterisation of the tumour is critical. If highest radiation doses are delivered in a targeted fashion, the chance of tumour cell kill increases and tumour control probability is enhanced.