The European Network for Cell Imaging and Tracking Expertise (ENCITE)
Since June 2009, the focus of research in the European Network for Cell Imaging and Tracking Expertise (ENCITE) has been on finding biomarkers to aid cell transplantation. Funded with €11 million from the European Commission (EC), this major project that runs until 2013, involves 10 countries. Their work is coordinated by the European Institute for Biomedical Imaging Research (EIBIR) network, founded by ESR Research Committee Chairman Professor Gabriel P Krestin, Chairman of the Radiology Department at Erasmus University Medical Centre, in Rotterdam.
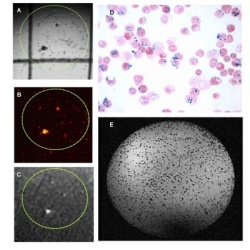
contrast agents.
A-C: Stem cells in a culture dish labelled
with fluorescent nanoparticles containing
Gd-DTPA, imaged by light microscopy (A),
fluorescent microscopy (B) and MRI (C). The
Gd-DTPA give rise to signal enhancement in
MR images.
D: MRimage of stem cells in a culture dish
labelled with iron oxide particles. The iron
oxide particles give rise to signal voids on
MR images.
Speaking with Daniela Zimmermann, Prof. Krestin explained that ENCITE had an unconventional beginning: ‘We actually achieved something unique in the history of health research projects that are subsidised by the EC -- we put out a competitive call after we started the project, whilst adhering to the financial budget we were given. Another 12 groups then responded to this and, with an independent committee, we selected the six groups that best complemented our expertise. We now have 27 partners in the consortium.’
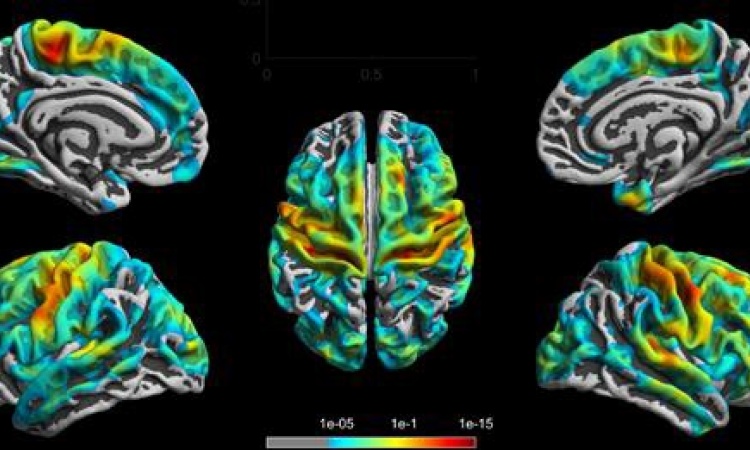
The project aims to make cells visible for therapeutic purposes with the help of markers, he explained. ‘So, first we have to develop the best possible labelling methodology, so that we can introduce standardisation, and then we have to capture the markers with suitable imaging modalities. Up to now, this has not been possible with standard imaging procedures. MRI scanning appears to be the most promising for the purposes of biomedical imaging, and we have already succeeded in cell-marking with the help of MRI in-vitro.
MR-PET probably is the most suitable, but we want to achieve the high sensitivity and resolution only with the MRI scanner. Our project is geared towards developing procedures that can really be utilised in clinical practice. Also, MR-PET was not available when we submitted our project three years ago. It is still at the experimental stage, so cannot yet be transferred into clinical use.’
Pointing out the various areas of application for cell therapy, he said: ‘From the treatment of tumours with antibodies to cardiovascular disease and diabetes – everything is possible. We have to distinguish between cell transplantation as an active drug, a drug delivery vehicle, or as a replacement for degenerated tissue. In the musculoskeletal field, in particular, it’s possible to treat cartilage or vertebral disc defects using tissue engineering with stem cells.
‘There have already been successful human experiments in insular cell treatment research, such as pancreatic cell transplantation in diabetics. In the case of transplantation of stem cells, or muscle cells into the myocardium, for instance, research has not yet progressed to that stage. Stem cell therapy without marking is already available for treatment after cardiac infarction. However, we want to make the defective cells and their possible regeneration visible, because stem cell transplantation into the myocardium has not yet been extensively researched and, moreover, remains controversial. We do not know how exactly these cells work – why they improve or worsen cardiac function. We assume it’s a hormonal reaction, and this is what we’d like to find out. We hope to be in a position to carry out the first human experiments in this area by the end of the project, in two and a half year’s time.’
Why are no commercial companies involved in this? ‘Our intentions are scientific, not economic. The global players have sufficient financial means to advance their own technical developments, but the industry is mainly interested in further developments in imaging technology. In our research MRI technology is only one of many areas of interest. The main issue is, and will remain, the development of the right contrast media probes required for cell labelling.
‘Even with the development of new markers we are initially doing experimental research. This results in two problems that puts companies off: The strict licensing procedures and the specificity of our probes. Molecular imaging procedures make it possible for us to develop specific markers that are customised for patients and their diseases. This means these are not “blockbuster” products that can generate huge profits. The cost benefit does not add up for industry –most of the money required during development goes towards the high licensing costs. This is why we will have strategic talks with contrast media manufacturers shortly, to tackle these problems jointly. Limitations posed by the licensing guidelines for contrast media are a big problem holding back the development of nuclear medicine. We are also likely to encounter difficulties within the context of our own project when it comes to the use of markers in humans. Modernisation and simplification of the current laws is of the essence here.’
The ENCITE project
The key work is carried out within sub-projects (SP), representing the expertise of participating groups:
SP-1: Novel Imaging Technologies. Lead: Prof. Jürgen Hennig, Medical Physics Department of Diagnostic Radiology, University Freiburg, Germany. Development of new pulse sequences and image post processing tools
SP-2: Novel Imaging Reporter Probes. Lead: Prof. Silvio Aimer, Chemistry Department and Centre for Molecular Imaging, University of Torino, Italy. Covering known exogenous probes and newly developed probes.
SP-3 - Novel Tools for Cell Labelling. Lead: Prof. Michal Neeman, Department of Biological Regulation, Weizmann Institute of Science, Israel. Assessing optimal labelling strategies to achieve maximum incorporation of the probe without causing adverse effects.
SP-4 - Pre Clinical Validation. Lead: Dr Monique Bernsen, Department of Radiology, Erasmus MC, University Medical Centre Rotterdam. Cell application and tracking strategies by means of different in vivo imaging techniques.
SP-5 - Translation towards Clinical Applications. Lead: Prof. Carl Figdor, Nijmegen Centre for Molecular Life Sciences, Radboud University, the Netherlands. Translation into clinical applications in dendritic cell (DC)-based vaccination, cell therapy for cardiovascular/ischaemic heart disease and pancreatic islets transplantation.
SP-6 - Dissemination and Training. Lead: Prof. Gabriel Krestin, Chairman of the Department of Radiology at ERASMUS University Medical Centre, Rotterdam. Creating awareness and the implementation of results to all stakeholders inside and outside the consortium.
06.03.2010