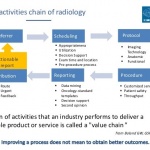
News • Defibrillator use
Italian law must change to improve survival from cardiac arrest
An Italian law requiring citizens to hold a certificate to use a defibrillator must change to improve survival from cardiac arrest, researchers argued today at Acute Cardiovascular Care 2018, a European Society of Cardiology congress. “Automated external defibrillator (AED) use before the arrival of the emergency medical services (EMS) plays a key role in improving victim survival from…