News • Immune system overreaction
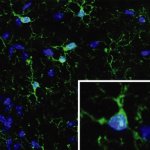
Sepsis can cause long-term damage in the brain
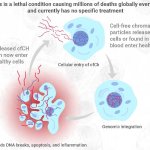
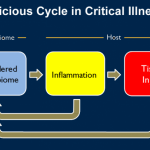
Infections can trigger a particularly strong immune reaction of the body (termed sepsis). In such a sepsis the immune system reacts so strongly that not only the pathogens but also tissues and organs are damaged. In a study with mice, researchers from the Technische Universität Braunschweig were able to show that sepsis can have long-term effects on the brain and learning behaviour even after…