Image source: Metalle-w, Ru1 modi, CC BY-SA 3.0
News • A BOLD-100 approach
Novel metallodrug shows promise in tumour treatment
BOLD-100/KP1339 is a ruthenium-based anticancer agent that has been co-developed at the University of Vienna and which has shown promising results in clinical trials in cancer patients. However, the mode of action of this metal compound has not yet been fully elucidated.
Researchers from the University of Vienna and the Medical University of Vienna have now been able to demonstrate that BOLD-100 binds to ribosomal proteins in tumour cells. The study now published with a cover in "Angewandte Chemie" can support a more targeted application of BOLD-100 as tumour-inhibiting active agent.
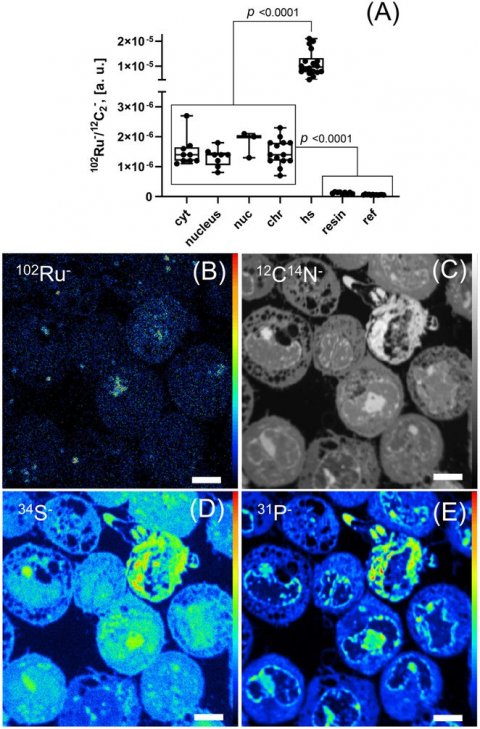
Image source: Neuditschko et al., Angewandte Chemie 2021 (CC-BY 4.0)
"In preclinical studies, BOLD-100 had excellent tumour-inhibiting effects in cancer types that weakly respond to platinum-based agents. We also know that the drug candidate creates stress in the endoplasmic reticulum, which constitutes a subunit of the cell that regulates lipid anabolism and protein maturation. However, there was a lack of understanding about the detailed mode of action of the active agent, especially with regard to potential target molecules to which the compound can bind and thus trigger the observable anticancer effects," say study authors Samuel Meier-Menches and Christopher Gerner from the Department of Analytical Chemistry. In cooperation with researchers from the Medical University of Vienna, they investigated in detail the molecular mode of action of BOLD-100.
Based on a multi-omics approach (a combined data analysis of the proteome and gene expressions), the researchers showed that BOLD-100 disturbs ribosomal constituents in the cancer cells, resulting in stress in the endoplasmic reticulum. "We were also able to identify potential protein binding partners of BOLD-100, more specifically the ribosomal proteins RPL10 und RPL24. Imaging techniques confirmed our results," says first author Benjamin Neuditschko from the Faculty of Chemistry, University of Vienna. "A comprehensive understanding how these active agents work enables us to use them in a targeted manner," the researchers conclude in their study.
Post-genomic approaches to investigate modes of action are one of the main goals of the Joint Metabolome Facility, a joint research platform of the University of Vienna and the Medical University of Vienna. The discovery and further development of metal-based agents for tumour types that were previously untreatable or difficult to treat are a main focus of the research cluster Translational Cancer Therapy Research, led by Bernhard Keppler (Faculty of Chemistry, University of Vienna) and Walter Berger (Medical University of Vienna).
Source: University of Vienna
08.03.2021