Article • Glioma detection
Breakthrough liquid biopsy test to detect mutations in brain tumours
Researchers at Massachusetts General Hospital (MGH) in Boston have developed a novel blood test using an enhanced form of liquid biopsy capable of detecting the most common types of genetic mutations that occur in glioma brain tumors.
Report: Cynthia E. Keen
Image source: Shutterstock/Alex Mit
The test is easy to use, inexpensive, produces results rapidly, and can be performed in most clinical laboratories. The researchers believe that the blood test has the potential to substantially improve the diagnosis and monitoring of gliomas.
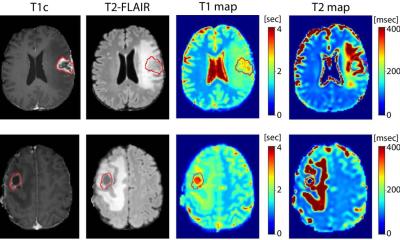
Gliomas, which originate in the glial cells that surround and support neurons in the brain, make up 81% of malignant brain tumors in adults, according to the National Brain Tumor Society. Liquid biopsy looks for fragments of tumor DNA that circulate in blood. It is a less invasive method than tissue biopsy for detecting cancer, and has been shown to be sensitive at detecting the presence of some forms of cancer. However, brain tumors have posed a formidable barrier for liquid biopsy, because mutant DNA is shed into the bloodstream at much lower levels than any other types of tumors.
The MGH-developed novel digital droplet polymerase chain reaction (ddPCR) assay can simultaneously detect and monitor two promoter mutations (C228T and C250T) in plasma samples of patients over time. These mutations, known to promote cancer growth, are present in more than 60% of all gliomas, and in 80% of all high-grade gliomas.

Co-principal investigators Leonora Balaj, M.D., and Professor Bob S. Carter, M.D., also of Harvard Medical School, told Healthcare in Europe that the assay took about a year to develop. They tested its sensitivity in 157 patients with TERT mutant or wildtype gliomas who underwent surgery at either MGH or Washington University St. Louis with age-matched healthy patients. The test achieved a sensitivity of 62.5% and a specificity of 90% in 114 patients with glioma who had had a standard tissue-based test to detect TERT mutations. This was tenfold improvement over any prior assay used to detect TERT mutations in the blood.
Writing in Clinical Cancer Research, the MGH Department of Neurosurgery researchers describe several technical optimization strategies they used to detect low concentrations of TERT promoter mutations in plasma. They acknowledge that although tissue biopsy will continue to be the gold standard for molecular diagnosis of glioma, their test may have two immediate applications. When a patient has a suspected malignant tumor in deep or inaccessible locations of the brain, the combination of the liquid biopsy test and MRI could be used with confidence. The liquid biopsy test could also be used to monitor the disease and the effectiveness of treatment, thus minimizing the risks caused by repeated brain biopsies.
We hope the same test can be used for liver, bladder, thyroid cancer and others cancers that harbor TERT mutations
Leonora Balaj
The researchers suggest that liquid biopsy, which effectively samples multiple tumor regions as blood perfuses the solid tumor mass, may ultimately become the gold standard for diagnosis. It could be a more sensitive indicator to detect TERT promoter mutation than a single focal tissue biopsy in some patients due to the heterogeneity of brain tumors. “We currently are focusing on getting the test CLIA validated so we can perform clinical utility studies,” Dr. Balaj told HiE. “We want to know the exact clinical scenarios where this test can be used and how clinical decisions can be made -- or changed -- based on this test. Increasing the sensitivity will require more basic research such as looking for more PCR stabilizers, higher volumes of plasma, and others.”
The researchers are also optimizing a test for another mutation (IDH1), to enable both tests to be used simultaneously to diagnose and stratify patients with glioma. Dr. Balaj said they hope to partner with other institutions to increase the number of plasma samples available, because clinical utility studies will require hundreds of samples. "We anticipate that this assay will also offer a sensitive method for detecting mutations in plasma cfDNA in other TERT positive tumors,” she added. “We hope the same test can be used for liver, bladder, thyroid cancer and others cancers that harbor TERT mutations.”
08.01.2021