
The therapeutic potential of adult stem cells in cardiovascular diseases
By Bodo E. Strauer (Düsseldorf, Germany) Russian summary and complete article in English.

By Bodo E. Strauer (Düsseldorf, Germany) Russian summary and complete article in English.
The Vestfold County Study, performed by Norwegian researchers, is the first large scale study comparing the results of a particular full-field digital mammography system (FFDM) with that of conventional mammography. Einar Vigeland, Department of Radiology, Vestfold Hospital, Tønsberg, Norway, and his colleagues collected data over a period of two years.

Cordis Corporations' CYPHER stent is a Sirolimus-eluting coronary stent used for treatment of patients with coronary artery disease. The five-year results of the E-Sirius Trial show that in comparison to bare metal stents the CYPHER stent offers sustained clinical benefits.
Researchers from the Robert Gordon University in Aberdeen, UK, presented a study at The British Pharmaceutical Conference, held from 10th to 12th September, 2007, at Manchester Central, showing that a chemical coating is able to encapsulate and protect insulin against enzymes that usually break down the hormone in the gastrointestinal tract.

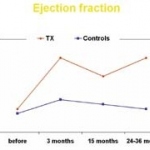
An increased TWA (T-wave alternans) is a significant indicator of all-cause and cardiovascular mortality, as well as of sudden cardiac death in patients with mostly normal ejection fraction, according to a recently published study by researchers led by Dr Tuomo Nieminen.
Cardiac infarction and cardiovascular failure are two of today's most frequent emergencies. SCHILLER's FRED® easyport® pocket is the only pocket defibrillator in the world.
More women than men die of cardiovascular disease (CVD) every year, yet females receive only 33% of angioplasties, stents and bypass surgeries; 28% of implantable defibrillators, and 36% of open heart surgeries.
The first implant of the Reveal XT, an insertable cardiac monitor made by US firm Medtronic, which recently received CE (Conformité Européenne) Mark, was carried out in June by Professor Karl-Heinz Kuck MD, at the Asklepios Klinik St. Georg in Hamburg, Germany.

Progenitor cell transfer to repair the damaged heart has emerged as an innovative and promising recent development in cardiovascular medicine.
Cardiac infarction is characterised by tissue ischaemia with loss of contractile heart muscle.

Switzerland - A clinical trial of cardiac resynchronisation therapy (CRT) in patients with advanced heart failure and a narrow QRS complex

A new blood pressure (BP) measuring device that provides, along with all the conventional cardiovascular parameters, the cardiac stroke volume, peripheral resistance and arterial augmentation, has been developed at the Austrian Research Centre (ARC), Vienna-Seibersdorf. The result of seven years' work by researchers, the device, named CardioMon, is now ready for sale.

UK — A report on the care of young surgical patients has been launched by The Children's Surgical Forum, a body of representatives from the medical royal colleges, surgical specialist associations, Department of Health, Royal College of Nursing and the Royal College of Surgeons Patient Liaison Group.

Launched last year, the Wound Infection Institute (WII), supported by Smith & Nephew Wound Management, now has a 130-strong membership, which include leading clinicians and scientists working to understand more about wound infections and their control.
The German firm Hartmann reports that Hydrotul, its new hydrocolloid impregnated dressing, combines the benefits of conventional impregnated dressings with those of hydro-active wound dressings.
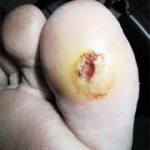
Diabetic foot syndrome (DFS) is one of the most serious sequela of diabetes mellitus - Disease management programmes (DMP) yield first results

Hutchinson's InSpectra StO2 Tissue Oxygenation Monitor - a portable device for treatment of trauma patients.

'The world at home in German hospitals' — thousands of wealthy foreign patients coming to boost budgets, this was the hope of many hospitals. Then along came a sobering study from Sozial und Seniorenwirtschaftszentrums GmbH (SWZ), conducted within the framework of Healthcare Export Projects, which are funded by the German Ministry for Education and Research to design, establish and market…

The Healthcare Inspection group has ordered 14 hospitals to stop surgery on the gullet to remove a tumour.

UK & France - The Hypertension in the Very Elderly Trial (HYVET), the biggest global clinical trial to assess the benefits of lowering blood pressure in patients aged 80+, was halted in July, two years before its scheduled completion in 2009.

An International Health Partnership: Richer countries urged to fast-track Millennium Development Goals for healthcare
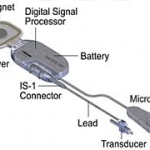
Otologics' Carina could improve the quality of life for patients suffering from hearing loss. The fully implantable hearing device uses a transducer to move the middle ear bones - much like the eardrum causes the middle ear bones to vibrate in response to sound wave. Carina implantation surgery takes about three hours and is an easy procedure with low surgical risks. (The whole article is only…
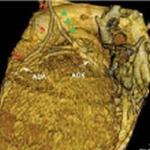
The aortocoronary bypass is an important surgical method for multivessel coronary revascularization, especially in the presence of complex lesions and in diabetic patients. It is can improve the prognosis of patients with three vessel disease and with left ventricular dysfunction1 . With regard to the type of graft used, bypasses are split into venous and arterial types. The use of venous…

The Swiss company Novartis recently received the FDA approval for the U.S. market for their new osteoporosis drug “Reclast”, a therapy that is only given once a year via a 15-minute infusion. The new treatment option is especially designed for women who suffer from post-menopausal osteoporosis.