FDA clears sale of patient-controlled ventilator
The US Food and Drug Administration (FDA) has given 510(K) clearance to Maquet Critical Care of Solna, Sweden, to market its Servo-i ventilator with the NAVA (Neurally Adjusted Ventilatory Assist) option. Sales of the system, which aims to treat and monitor neonatal, infant and adult patients, should begin this year. In addition, current Servo-i users can upgrade their system with NAVA.
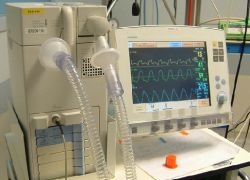
In this new approach to mechanical ventilation, to improve synchrony between patient and ventilator the patient’s own respiratory centre can control the ventilator. Signals from the brain’s respiratory control centre are transmitted through the phrenic nerve to the diaphragm, where a catheter captures the electrical activity (Edi) and feeds it to the ventilator. The ventilator responds by providing the requested level of support to the patient. As the ventilator and diaphragm work with the same signal, the coupling between the two is virtually instantaneous.
In addition to being a distinct mode of ventilation, NAVA also enables a complete evaluation of the neural respiratory control by capturing the electrical activity of the diaphragm (Edi). The Edi signal can be used as a unique monitoring tool, as it provides information on respiratory drive, volume requirements, the effects of ventilatory settings, and to gain indication for sedation and weaning.
Christer Ström, Director Ventilator Program, Maquet Critical Care, said: ‘We are proud to introduce the most progressive advancement within respiratory therapy since the introduction of mechanical ventilation 30 years ago.’
Details: www.maquet.com
01.05.2007