News • Faster diagnosis, reduced cost
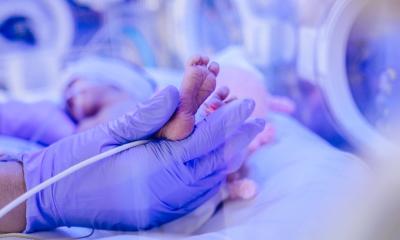
The impact of whole genome sequencing on newborn babys in ICU
Early whole genome sequencing might bring hope for children who are born severely ill or who develop serious illness in the first few weeks of their life.
Because these children are often difficult to diagnose, detection of diseases has considerable implications for their short and longer-term care. At the annual conference of the European Society of Human Genetics (ESHG) in Milan, Italy, the researchers presented their findings on how whole genome sequencing carried out quickly has the potential to provide an early diagnosis, and thus improve the clinical care of these infants as well as reducing costs. Dr Shareef A. Nahas, Senior Director, Rady Children’s Institute for Genomic Medicine, San Diego, CA, United States, reported on his team’s study of rapid whole genome sequencing (rWGS) of all inpatient children under one year of age who were nominated for genetic investigation at Rady Children’s Hospital. Rapid WGS is able to return results in 48 to 96 hours, whereas standard genetic testing takes six to eight weeks to provide a result. They then noted subsequent changes in medical care that occurred while the child was still in hospital. Where there was a significant change in care due to a new diagnosis, the cases were reviewed by an independent expert panel who tried to determine what they believed would have happened had the child not received rWGS.
"Personalised genomic medicine is becoming a reality!”
There is an ethical imperative to act in the best interest of neonates, but implentation will require a concerted effort across all healthcare systems
Shareef A. Nahas
After 12 months of testing, 363 patients had been enrolled in the study and rWGS interpreted in 340 of them. This yielded a diagnosis in 115 cases (about 34%). Diagnosis occurred quickly, on average within 96 hours. Changes in management as a result of diagnosis were identified in 77 patients, or about 67% of those diagnosed. Such changes ranged from specific changes, for example surgical interventions, to guidance in palliative care. Among the first 42 infants diagnosed, rWGS provided over $1.3 million in net cost saving over the projected standard care. "To date, our studies have shown a considerable clinical and economic benefit of sequencing children who were identified by clinicians as being suspected of having a genetic disorder. In the course of the study, one child was spared devastating neurological damage, and one had a significantly reduced risk of death. The net cost savings totalled several hundred thousand dollars, even when we included the cost of analysing the genome of the child and both parents," says Dr Nahas.
Although many studies have shown that WGS improves the diagnosis if genetic disorders in infants and can lead to beneficial changes in their management, the new research has shown that, by implementing rapid sequencing, cost savings will also ensue. "We are now in a situation where we have a technology that leads to improved diagnosis and improved outcomes but is also not a net burden on healthcare resources. This means that for large healthcare payers, there is not a logical cost barrier to implementing rWGS in neonates suspected to have a genetic disorder. There will need to be further data on who else can benefit from early use of this technology but implementation in the current cohort should not be delayed," says Dr Nahas.
Currently, the use of WGS among sick neonates is very infrequent across the world, and there are few healthcare systems that have the ability to turn round genetic testing quickly enough to be clinically relevant, the researchers say. This is vital if medical management needs to be changed during the childrens’ hospitalisation. In the course of Dr Nahas’ study, one child was spared devastating neurological damage and another had a significantly reduced risk of death.
"The logic for the use of rWGS in these patients, both diagnostic and economic, is totally convincing. We have demonstrated that early sequencing saves money during admission. We were surprised by the proportion of children who received a change in care during that admission – around 25% of children sequenced and 80% of those diagnosed. This rate is much higher than other published rates for neonates who received WGS. We believe that this difference is due to the fact that the children received results at a much younger age, at a point where medical decisions were yet to be made. There is an ethical imperative to act in the best interest of neonates, but implentation will require a concerted effort across all healthcare systems, and this will need to be at government level in Europe. Consistent with many diagnostic tests in the post-natal period, rWGS has the potential to identify conditions associated with lifelong disability or shortened lifespans," Dr Nahas concluded.
Chair of the ESHG conference, Professor Joris Veltman, Director of the Institute of Genetic Medicine at Newcastle University, Newcastle, United Kingdom, said: “This study confirms the value of genome sequencing to detect the cause of unexplained disease. It shows that this can now even be done within four days, which is very impressive. This greatly increases the practical use of genetics in an acute clinical setting where treatment decisions can now be made based upon this powerful test. Personalised genomic medicine is becoming a reality!”
Source: The European Society of Human Genetics
18.06.2018