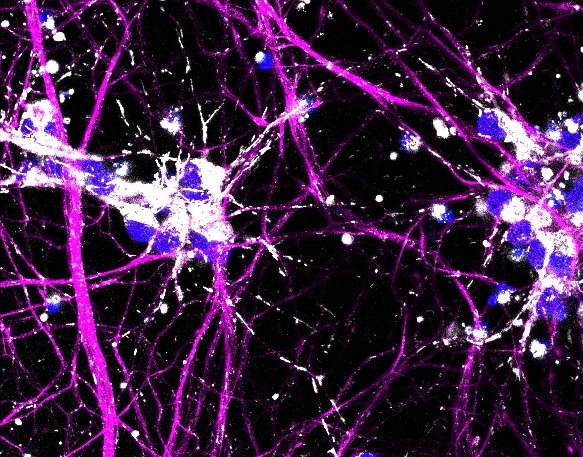
Image source: Stockholm University; photo: Melanie Leboeuf
News • Amyotrophic lateral sclerosis
New insights on ALS onset opens paths to earlier treatment
Using the gene scissors CRISPR and stem cells, researchers at Stockholm University and the UK Dementia Research Institute (UK DRI) at King’s College London have managed to identify a common denominator for different gene mutations that all cause the neurological disease ALS.
The research shows that ALS-linked dysfunction occurs in the energy factories of nerve cells, the mitochondria, before the cells show other signs of disease, which was not previously known. The study was recently published in the scientific journal Nature Communications.

Image source: Stockholm University, photo: Sören Andersson
“We show that the nerve cells, termed motor neurons, that will eventually die in ALS have problems soon after they are formed. We saw the earliest sign of problems in the cell’s energy factories, the mitochondria*, and also in how they are transported out into the nerve cells’ long processes where there is a great need for them and the energy they produce,” says Dr Eva Hedlund at Stockholm University, head of the study together with Dr Marc-David Ruepp at the UK Dementia Research Institute at King’s College London.
The research team was able to establish that these problems were common to all ALS-caused mutations, which will be important for future treatments of the disease. “This means that there are common factors that could be targeted with drugs, regardless of the cause of the disease,” says Dr Eva Hedlund.

Image source: Stockholm University
The researchers used the gene scissors CRISPR/Cas9 to introduce various ALS-causing mutations into human stem cells, called iPS cells*. From these, motor neurons, the nerve cells that are lost in in ALS, and interneurons, nerve cells that are relatively resistant to the disease, were produced. These were then analyzed with single-cell RNA sequencing, a method that enables identification of all messenger molecules (mRNA) in each individual cell and with that understand how a particular cell works, how it talks to its neighbors and if it starts to have problems.
“In the data we obtained, we identified a common disease signature across all ALS-causing mutations, which was unique to motor neurons and thus did not arise in resistant neurons,” says Dr Christoph Schweingruber, first author of the study.

Image source: UK DRI
This happened very early and was completely independent of whether the disease-causing mutated proteins (FUS, or TDP-43) were in the wrong place in the cell or not. “Until now, it has been believed that it is the change where the proteins are within the cells, called mislocalization*, that occurs first,” says Dr Marc-David Ruepp.
In ALS, it is often said that some problems are caused by a loss of function in a protein that is mutated, while other problems arise due to the opposite, namely the emergence of a new toxic function that has been obtained through the mutation, called “gain-of-function”, but according to Eva Hedlund, it has not always been easy to clarify how it really works and much is still unknown. “By making various CRISPR mutations in the ALS-causing FUS-gene*, we have now been able to show for the first time that most errors arising are caused by a new toxic property of the protein, not by a loss of function,” says Dr Christoph Schweingruber.
A third discovery was that the transport of mitochondria out into the axons*, the extensions of the nerve cells where most mitochondria in nerve cells are needed, was radically affected in the ALS lines. This happened independently of whether the disease-causing proteins were in the wrong place in the cell or not. “A fact that poses a problem because there is a great need for these energy factories in the extensions of the nerve cells. Without them the nerve cells do not have enough energy to communicate properly with other cells,” says Dr Eva Hedlund.
The new discoveries open up for early treatment methods, something that for the research team is a continuous work in progress. “We are trying to understand how these early errors occur in the sensitive motor neurons in ALS, and how it affects energy levels in the cells and their communication and necessary contacts with muscle fibers. We believe that these are important keys to the understanding of why the synapses between motor neurons and muscles is broken in ALS and also to identify new targets for therapies,” says Dr Eva Hedlund.
Source: Stockholm University
22.05.2025