Article • Improving quality and efficiency
Preparing for AI in clinical laboratories
Some year in this decade, AI tools will become ubiquitous within clinical laboratories. AI has the potential to increase the accuracy of laboratory testing and improve the quality and efficiency of operations and service of testing labs.
Report: Cynthia E. Keen

Clinical laboratorians must prepare to help lead this initiative, for their knowledge will be the key to successful implementation. They need to learn how AI algorithms are developed and validated, how to justify and analyze impact from the perspective of clinical laboratory medicine, and how to implement them to best benefit the patient and the hospital. Several scientific sessions at the 2023 Association for Diagnostics and Laboratory Medicine (formerly the AACC) Annual Scientific Meeting focused on this topic.
AI is in its naissance in medical lab development, and this was very apparent in the technical exhibition of the ADLM/AACC meeting. David McClintock, MD, Chair of the Division of Computational Pathology and AI in the Department of Laboratory Medicine and Pathology of Mayo Clinic, pointed out that only 30 out of the 941 exhibitors at the meeting included the terms 'artificial intelligence' (AI) and/or "machine learning" (ML) in their product/company descriptions on the AACC exhibitor website. Ten companies included "analytics" in their description, but only four were separate analytics-based companies selling clinical lab AI/ML software. 'This is an emerging space, just as radiology PACS was 30 years ago,' McClintock said. 'Now is the time to learn about it or perhaps even start to developing models that can benefit your lab.’
Uses of AI/ML in the lab
There are numerous ways that both simple and complex AI tools can aid a clinical laboratory. These include:
- Automated spectroscopic data analysis and disease detection; multivariate analysis of disease conditions; test interpretation;
- Digital image analysis for microbiology, haematopathology, immunology, and forensics;
- Data entry automation for specific tasks and processes;
- Creating standardised reports for lab test results and automated entry into Laboratory Information System (LIS);
- Minimising laboratory testing for inappropriate test orders, predicting test results from other available data on patient chart, and reducing redundancy and duplication of lab tests, based on prior type and date of tests already performed;
- Data analytics for laboratory operations planning, such as predicting volume workflow, employee staffing requirements, etc.;
- Identifying and alerting for abnormal test results;
- Auto-verification of test results for quality control.
Automating spectral analysis for kidney stones and fecal analysis
Mayo Clinic spent six years developing an AI model to automate the spectral analysis of stones passed by patients. It is based on the classification of 708 unique kidney stone types. In the first 90 days of implementation, commencing April 2023, 20,000 stones have been reviewed, 40% of which were newly able to be bulk auto-released to patients’ medical records.
Before the model was implemented, the conventional workflow began with cleaning and drying the stone, after which it was ground into a fine powder and manually analyzed with FTIR spectroscopy. A technician manually entered the results into a LIS, followed by a second technician reviewing the interpretation. Only then are results uploaded to a patient’s electronic medical record (EHR).
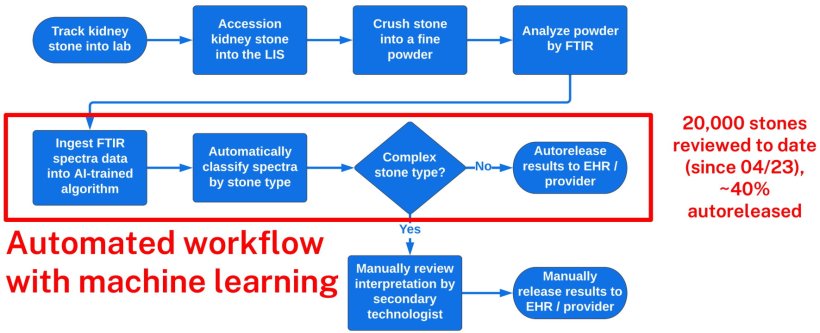
© ADLM
AI tool to automate multiple processes
After evaluating how AI could improve workflow, reduce costs, and increase efficiency, the Kidney Stone lab at Mayo Clinic Rochester, in conjunction with an innovative AI team from Mayo Clinic Florida, created an AI tool to automate multiple processes following FTIR spectra analysis. The AI model was trained on 70,000 kidney stone spectra and validated with 16,491 kidney stone spectra. Quality assurance required 81,517 kidney stone spectra.
'This is a lot of data, which took a lot of work, and a lot of computing time,' commented McClintock. 'But now the process is automated and is achieving our expectations. If a stone is not complex, the AI system classifies it and then the information is automatically entered into the LIS and subsequently released to the patient’s EHR. When it identifies a stone as complex, the results produced are manually flagged for review by a technician. The lab is now saving a lot of time, which equates to tangible cost savings and opportunities for laboratory staff work reduction/redirection.’
Other applications of AI in the clinical labs also exist, such as commercially developed AI tools in clinical microbiology to detect fecal ova and parasites (O&P). For most labs, up to 95% of O&P cases can be negative, and thus the process of reading slides can be monotonous. The investigational AI-assisted screening tool (Techcyte, Orem, UT) uses a convolutional neural network to identify and count parasite cysts and trophozoites, yeast, and red and white blood cells and groups them by class. Techcyte claims the tool is five times more sensitive than manual examination, with a sensitivity of 98.9%. It produces findings within 30 seconds instead of the average five minutes, automatically uploading negative findings to a LIS. Positive samples are flagged for technologist review and assessment. At Mayo Clinic, this test has just been implemented, with initial impressions positive by laboratory staff who can now remotely review slides, improving employee experience.
What clinical lab managers need to think about
'Don’t get enamoured with AI for your lab,' McClintock cautioned. 'Always remember a clinical lab’s primary objective: to deliver the right information to the right person at the right place and right time in the right way. No system today can integrate all potential outputs of AI tools,' he emphasized. 'LIS, EHR, and middleware solutions take considerable effort to integrate with any AI tool without encumbering the pathologist, laboratorian, or clinician. For starters, you need to think about data governance, data pipelines, regulatory guidelines, ethical review, custom programming and coding, computational computing power, either locally or in the cloud, cybersecurity, and risk management.
I think that AI will be another tool in our tool bag, to aid in efficiency and quality control
Christopher Lee Williams
‘Don’t forget the cost and availability of AI maintenance, support and quality control, which all require new IT support skills. There are also AI specific tools, such as algorithm drift. And then, in the end, will the AI tool save you enough money or at some point be reimbursable so that it can pay for itself?’
Experts recommend that lab managers need to focus on generating clinical evidence for AI benefits and understand the barriers and challenges to implementation when they select a tool. New training on AI is essential. In general, experts recommend implementing the new tool while still keeping the existing system functioning, to give practitioners time to learn and get comfortable with AI while maintaining the status quo as back-up.

McClintock also described a new framework for clinical AI life cycle implementation, from idea generation to final validation, go-live, and system maintenance. By adopting a similar AI lifecycle, he encouraged attendees to embrace the potential of AI in their labs.
Co-presenter Christopher Lee Williams, MD, Assistant Professor of Pathology at the University of Oklahoma Health Sciences Center, concurs. 'Will AI be replacing staff in labs?' he asked rhetorically. 'Probably not. The practice of laboratory medicine has been constantly evolving, often due to increasing automation. I think that AI will be another tool in our tool bag, to aid in efficiency and quality control. With a steady increase in an aging population in the United States, we are going to need all the help we can get.'
Profiles:
David McClintock, MD, is the Chair of the Division of Computational Pathology and Artificial Intelligence within the Department of Laboratory Medicine and Pathology at Mayo Clinic in Rochester, Minnesota, USA. His primary clinical interests include clinical informatics, laboratory workflow optimisation, digital pathology implementation, analytics, and clinical ML/AI model deployment. His research interests include understanding the role and effects of digital pathology within the clinical laboratories and the use of AI and ML for improved diagnostics, more efficient workflows, and better patient outcomes.
Christopher Lee Williams, MD, is the Director of Informatics in the Department of Pathology at the University of Oklahoma Health Sciences Center in Oklahoma City, where he also serves as an Assistant Professor. Dr Williams’ current research interests include how to operationalise AI tools for analysis and reporting in the laboratory setting, and in optimizing UI/UX design for laboratory workflows.
18.03.2024