News • Non-invasive
New device for thyroid cancer screening
The international LUCA consortium has developed a non-invasive, low-cost device that brings a new solution for thyroid cancer screening. Funded by the European Commission, the device aims to attain more precise and accurate diagnosis of thyroid nodules to help reduce unnecessary surgeries.
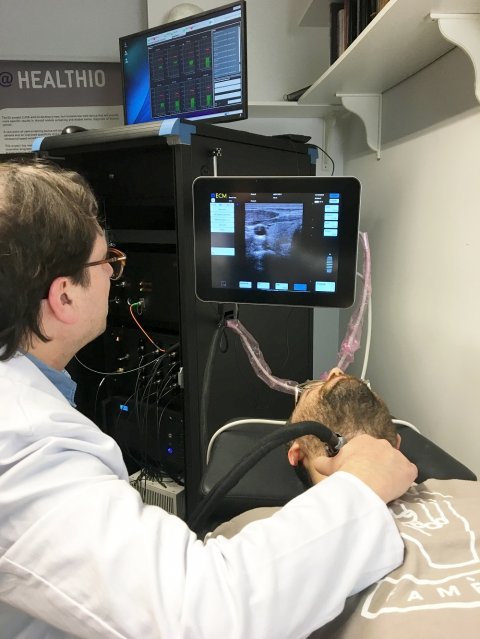
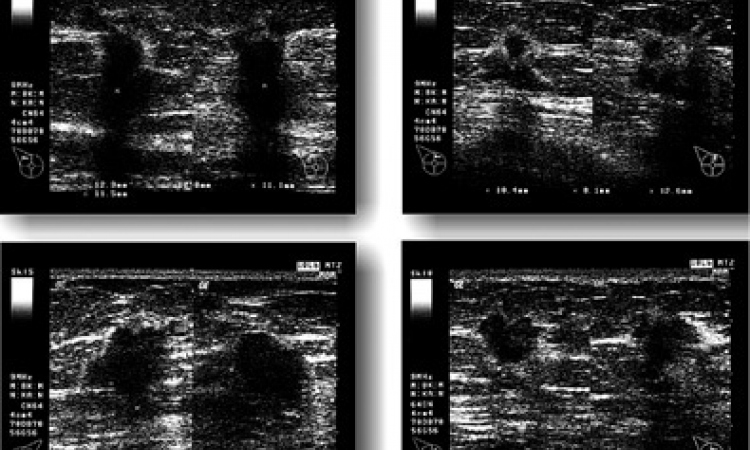
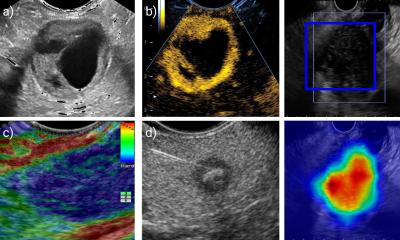
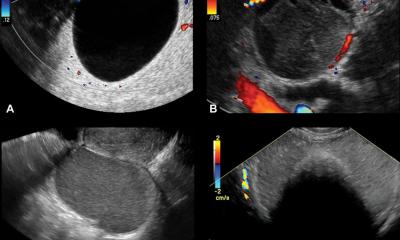
Thyroid cancer is a major health issue for European citizens, with about 50,000 new cases diagnosed each year. The methods used nowadays to detect and diagnose thyroid cancer do not provide physicians with the adequate tools to accurately determine whether the thyroid nodules are benign or malignant. Thus, false positive results cause a high number of unnecessary surgeries, which not only account for major health costs to European hospitals but also produce needless discouragement on patients and their families.
In search of improving sensitivity and specificity of screening processes, the four-year EU-funded project Laser and Ultrasound Co-analyzer for Thyroid Nodules (LUCA) has developed an innovative, low-cost device for a more specific thyroid nodule screening and thus better diagnosis.
By combining the traditional multiparametric ultrasound imaging technique with a state-of-the-art photonic approach that obtains measurements of tissue hemodynamics and thyroid nodule composition with the use of light, the LUCA device will enhance the non-invasive characterization of the thyroid tissue, aiming to reduce the amount of uncertainty in the diagnosis of thyroid nodules. In a short video recently released by the LUCA consortium, the researchers explain the science behind LUCA.
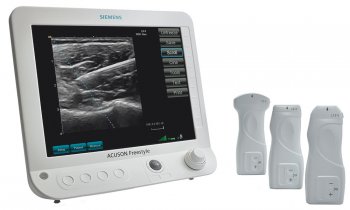
After three years of hard and intensive work, the international and interdisciplinary team of clinical endocrinologists, radiologists, physicists, and engineers, together with industrial partners, have now realized the first LUCA prototype and reached the final phase of their ambitious endeavour: testing the device’s performance in humans. “With the LUCA prototype ready for clinical testing, we have completed a major milestone in our project. The study in humans will help us to validate and refine the LUCA device. In the end, we expect that LUCA will help to significantly reduce the number of invasive procedures and enable improved clinical decision-making,” says Turgut Durduran, from ICFO- The Institute of Photonic Sciences (Spain) and Scientific Coordinator of LUCA.
The first in vivo human tests in January 2019 have validated the capability and performance of the device as well as the quality of the measurements. To accomplish the next step in the validation process, which is introducing LUCA into the routine processes for clinical use, a feasibility study will be conducted on healthy volunteers and patients at the Hospital Clínic de Barcelona.
Source: European Society of Radiology
27.02.2019