Video • Cancer research
3D-printed tumor model reveals metastasis mechanisms
Using 3D printing, scientists replicate the tumor environment to visualize cancer’s spread in real time
Scientists have created a 3D-printed model to mimic the specific conditions that spur the spread of cancer cells. The model, published in the journal Life Science Alliance, allows researchers to study a process previously hidden from view and may open the door to new screening and treatment options for cancers at risk of spreading.
Thanks to advances in prevention, diagnosis, and treatment, many cancer patients have good prognoses and are living longer. However, some tumors still spread to other organs throughout the body—a process known as metastasis—which makes treatment incredibly challenging. In fact, metastatic cancers—and not the original tumor—are responsible for most cancer deaths.
Recommended article
Article • Awareness
Focus on cancer
From solid tumors to metastatic carcinomas and leukemia: cancer is among the most common causes of death. Keep reading for latest developments in early detection, staging, therapy and research.
“Studying the moment where and when a relatively passive tumor cell acquires the ability to move and metastasize could be a game changer for cancer treatment,” said Carlos Carmona-Fontaine, an associate professor of biology at New York University and the study’s senior author. “Unfortunately, it is virtually impossible to directly witness this transition, and as a result, there are no therapies that target this critical but understudied step in the progression of cancer.”
The conditions we observe in the 3MIC may create an environment that protects tumors from at least some treatments, which may help us begin to explain why metastases are so difficult to treat
Carlos Carmona-Fontaine
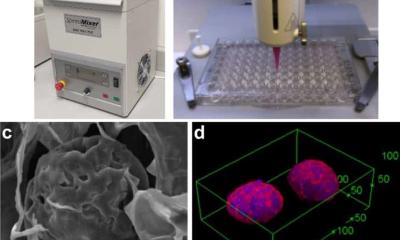
Most metastatic cells arise from crevasses deep within tumor tissues where oxygen and nutrients are scarce. This resource scarcity is essential in triggering metastases. However, because this scarcity happens in cells buried within hard-to-reach tumor regions, it is challenging to observe directly—in patients, animal cancer models, and even in other lab-based tumor models. The researchers decided to tackle this problem by building a tiny tumor model that replicates the specific conditions that promote the acquisition of metastatic properties in tumor cells. The model—which they named “3MIC” for the 3D microenvironment chamber—follows the evolution of malignant cells using live microscopy, which images cells in real time.
Using 3D printing technologies, the researchers designed the model with unique geometry to allow for imaging these deep and nutrient-starved cells with unprecedented detail. “One of the most important conditions in the emergence of metastasis—this lack of nutrients and oxygen—was also one of the most difficult to recreate and probably the most important innovation of the 3MIC,” said Carmona-Fontaine.
The 3MIC also allowed the researchers to add additional cells, such as macrophages and fibroblasts, that are known to partner with the tumor during the metastatic process. As a result, they were able to study how tumor cells migrate, invade, and interact with these other cells under different metabolic conditions.
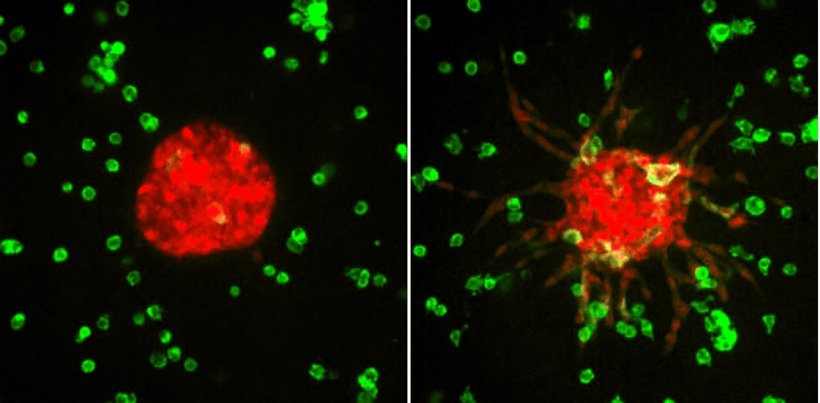
Image credit: Carmofon Lab/NYU
In their study, the researchers first confirmed that known metastasis-promoting factors, such as low oxygen, were also relevant in the 3MIC. Interestingly, their data not only confirmed this but also suggested a mechanism where low oxygen indirectly promotes metastasis through lowering the pH of the local tumor environment and making it more acidic.
The researchers also showed that drugs—specifically, versions of the chemotherapy Taxol—that effectively target tumor cells under normal conditions failed to act on the resource-deprived tumor cells, thus sparing them. This may suggest that metastatic cancers’ lower drug response may be due to intrinsic changes that make cells more drug-resistant, not lower drug concentration—a distinction that was previously difficult to measure. “In other words, the conditions we observe in the 3MIC may create an environment that protects tumors from at least some treatments, which may help us begin to explain why metastases are so difficult to treat,” said Carmona-Fontaine.
With the 3MIC in hand, the researchers are now focused on finding early signs of cancer metastasis before the cells spread, which could be used as a diagnostic tool to predict metastasis and to test possible therapeutic targets that could interrupt this process.
In addition to Carmona-Fontaine, study authors include Libi Anandi, Jeremy Garcia, Manon Ros, Libuše Janská, and Josephine Liu of NYU’s Center for Genomics & Systems Biology. The research was supported by the National Cancer Institute (DP2 CA250005), the American Cancer Society (RSG-21-179-01-TBE), the Pew Charitable Trust (00034121), and the National Institute of General Medical Sciences (T32GM132037-01).
Video at the top: Lung cancer cells grown inside the 3MIC over 72 hours. Tumor cells on the left were grown under normal conditions, while tumor cells on the right were grown in a nutrient- and oxygen-scarce region within the 3MIC, producing streams of migratory cells and a dramatic change in the shape of the tumor cluster. Credit: Carmofon Lab/NYU
Source: New York University
24.10.2024