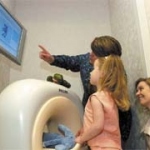
The Kitten Scanner
The Philips Kitten Scan is a miniature CT (or CAT) scanner for children - no, not to be scanned in it, but for them to use while awaiting checkups themselves.

The Philips Kitten Scan is a miniature CT (or CAT) scanner for children - no, not to be scanned in it, but for them to use while awaiting checkups themselves.

In 1982, ulrich medical - a 3rd generation family concern based in Ulm - presented the first contrast agent injector for CT examinations.

X-ray computed tomography (CT) has shown an absolutely remarkable and impressive increase in its performance characteristics for many years - remarkable because the modality was declared dead in the 1980s, impressive because these developments seemed impossible to many, for technical and for physics reasons.

The bucky table is an inexpensive tool for X-Ray departments. However, due to the increasing use of movable stands, especially combined with digital imaging receptors, further requirements for a patient positioning table arise.

England - The Thames Gateway Business Awards

Alfred E Schiller, founder and managing director of Schiller AG, based in Baar, Switzerland, describes the rise of his company and its place in today’s highly competitive intensive care market. Alfred Schiller founded Schiller AG in 1971 and three years later introduced his first product – a pocket-sized electrocardioscope, which has been built on successively over the years. The…

The Imaging Science Institute (ISI) — officially opened in December by Professor Werner Bautz, Director of the Institute of Radiology at the University Hospital Erlangen and Professor Erich R Reinhardt, member of the board at Siemens AG and chairman of the divisional board at Siemens Medical Solutions (Erlangen) — is the third institute (after Berlin and Tubingen) in which Siemens is…

I am delighted that the Hospital Administrator Symposium organised by European Hospital is being held in conjunction with the ECR again this year.
Following a 12-month examination of the way medical devices are monitored for safety after approval, the USA's Food and Drug Administration (FDA) has launched a new programme to `transform and strengthen´ current monitoring of new technology as well as existing products.

Formerly integrated with the electronics/medical division of ...
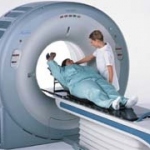
Following enthusiastic reactions to the Aquilion Large Bore (LB) scanner, when shown as a work in progress at last year's ECR, and with orders in hand, Toshiba has commenced production.

Bosch launched a new paging system which automatically diverts paging calls to mobile phones, so that an SMS message can be sent.

Around €18 billion annually is spent on medical technology in Germany. After the US and Japan, the German medical technology market is the third largest in the world.

In 2005, De Lage Landen financed (worldwide) medical equipment worth US$1 billion, by various methods such as loan, lease, rent and ppp, either directly with end-users or indirectly by refinancing manufacturers.
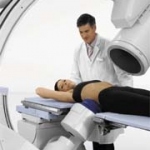
Lithoskop, a new multifunctional lithotripsy system, is to be publicly launched by Siemens Medical Solutions at the European Association of Urology Congress, to be held in Istanbul, Turkey this March.
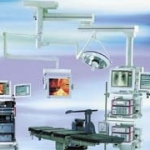
Core is a modular system that interacts with individual operating theatre (OT) devices to bring them under the ontrol of one centralised monitor.
Although parents are allowed in neonate ICU units, family members and friends are barred. To meet this need, Innsbruck Medical University recently launched the project 'Babywatch' in its neonatology ICU.

Forli, Italy - With construction almost completed, the 550-bed Ospedale Nuovo G B Morgagni can now provide state of the art services for the community it serves.
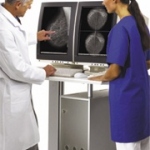
Mammography plays a critical part in diagnosing breast cancer. Although this does not prevent the disease, diagnosing breast cancer as early as possible can save lives.

Karlsruhe, Germany - A new type of ultrasound computed tomography (CT) system promising to improve diagnosis significantly is currently being developed at the Research Centre Karlsruhe.
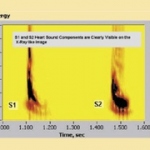
A processing system said to pick up cardiac sounds and correlate these with any related abnormalities, e.g. valve defects, stenosis, fibrillation, septal defect, etc, has been developed by the US firm Biosignetics Corporation.
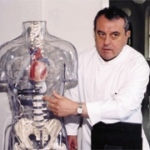
Ventricular assist devices (VAD) have been used since the 1980s, primarily to provide support after cardiac surgery for several days during recovery, or more often to keep patients alive until later heart transplantation (HTx).
The UK - In the 1990s, the nationally co-ordinated NHS Breast Screening Programme was already saving lives - a 21% fall in breast cancer mortality over the last decade and, with the cervical screening programme, this was viewed as among the best cancer screening programmes in the world. However, in that period, the country's cancer services, as a whole did not match up to those of other European…

Camena, an innovative ventilator that provides clinical-quality ventilation for patients at home, will be launched, at the European Respiratory Society (ERS) annual meeting (4-8 September 2004, Glasgow, UK), by Dräger Medical AG & Co KGaA, of Lübeck, Germany.
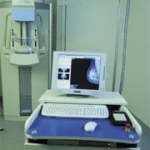
The Swedish firm Sectra reports that its digital MicroDose Mammography system reduces radiation by 80%, compared with traditional film-based systems, and that its completely new detection technology allows this without compromising image quality.