Distinguished by precision and accuracy
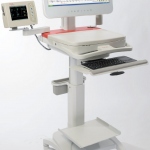
We are very pleased to introduce our new Exercise Stress Test System CARDIOVIT CS-200 Touch which can perform multiple clinical tests: 12-/16-channel resting and exercise ECG, Holter ECG and 24h ABPM.

We are very pleased to introduce our new Exercise Stress Test System CARDIOVIT CS-200 Touch which can perform multiple clinical tests: 12-/16-channel resting and exercise ECG, Holter ECG and 24h ABPM.

The highly-anticipated and positive results from the IN-TIME study of home monitoring technology were recently presented in a hotline session at this year’s European Society of Cardiology (ESC) congress by coordinating investigator Professor Gerhard Hindricks MD, from the Heart Centre, University of Leipzig, Germany.

Samsung BioLogics today announced that Samsung BioLogics and F. Hoffmann-La Roche have entered into a long term strategic manufacturing agreement under which Samsung will manufacture Roche's proprietary commercial biologic medicines at its two manufacturing facilities in Incheon, South Korea, of which one is currently under construction.


Quality management (QM) for medical laboratories shaped its footprint during recent years – it’s a hot topic in papers, conferences and trade fairs. A lot of information makes a lot of work, says Dr Markus Neumann

Canadian firm Labotix Automation Inc. has been acquired by the Cerner Corporation based in Kansas City, Missouri, USA, amid predictions that the combination of the Labotix’ solution with Cerner Millennium and Cerner Copath will create the most comprehensive set of capabilities on the market to support high volume testing.
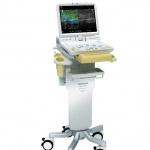
At ECR 2013 Hitachi Medical Systems Europe will for the first time in Europe be showcasing Noblus, an advanced versatile and compact ultrasound platform.

A prototype for proton and ion therapy has been used clinically in Heidelberg since 2009. Built jointly by Siemens and GSI (Helmholtz Centre for Heavy Ion Research) in Darmstadt.

The European medical technology industry association Eucomed notes with great concern recent press reports that the French firm Poly Implant Prothèse (PIP) may have violated the regulations by using unapproved industrial-grade silicone in some of its implantable products and by allegedly falsifying or withholding documents.

3M Health Care and Materials Management Microsystems recently announce plans to establish connectivity between Microsystems' SPM® sterile processing information system and 3M™ Attest™ Auto-readers and 3M™ Steri-Vac™ EO Sterilization Systems. This collaboration will provide hospitals more streamlined data collection, documentation and reporting capabilities.