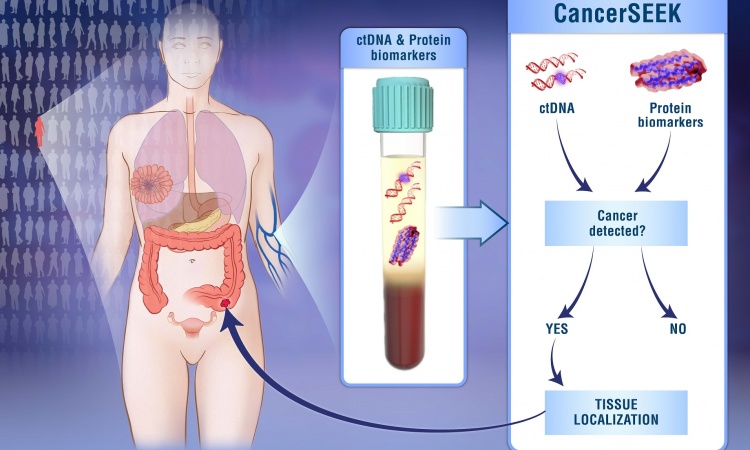
Lung Metastasis
Subpopulation of white blood cells stands guard
One goal of immunotherapy is to rally a patient’s often over-burdened immune cells to effectively attack a tumor. Among foot soldiers on the immune front line is a subpopulation of white blood cells called “patrolling monocytes,” whose job is to cruise the bloodstream, cart off cellular debris, and block invasion of a less benign population of inflammatory cells.
Previously, researchers knew these white blood cell scavengers played a beneficial role in counteracting inflammation in the context of atherosclerosis. Now, a study from the La Jolla Institute for Allergy & Immunology (LJI) illustrates that patrolling monocytes may also play an anti-cancer role, particularly in the lung. In it, Catherine C. Hedrick, Ph.D, and colleagues track the wanderings of these cells in the lung vasculature, film them pursuing invading tumor cells, and employ a mouse model that they developed to demonstrate how effective this pursuit is in a living animal.
That study is the first to show that that patrolling monocytes directly block lung metastasis, findings that could suggest future immunotherapies to treat an extraordinarily lethal cancer. “Our study shows that this subgroup of white blood cell is a key player in orchestrating the killing of metastasizing tumor cells,” says Hedrick, a Professor in the Division of Inflammation Biology. “These cells recognize and could help destroy early metastasizing tumor cells in the blood, even before they can invade new tissues and form new tumors.”
Hedrick’s group identified the requisite tool for the study in 2011, when they discovered that mice lacking a gene called Nr4a1 was required for monocyte survival. As reported then in Nature Immunology, Nr4a1 “knockout” mice appeared normal but specifically lacked patrolling monocytes. Richard Hanna, Ph.D., an LJI Instructor, is first author of both that paper and the recent Science paper. “At the time no one had looked at how these cells might function in cancer,” says Hanna. “The knockout mouse gave us a model to test what they do.”
That test involved injecting fluorescently tagged melanoma cells into the bloodstream of either normal or Nr4a1 knockout mice and following where tumor cells migrated. Within 24 hours, blood vessels in lungs of knockout mice contained more melanoma cells than did comparable vessels in normal mice, suggesting that the absence of these defenders made animals vulnerable to lung metastasis.
The group then conducted what biologists call a “rescue” experiment, meaning they transfused patrolling monocytes from normal mice into the bloodstream of knockout mice before injecting tumor cells. That intervention inhibited cancer cell migration into lung vasculature, a finding Hanna cites as critical: “The fact that you can add these cells back into a mouse that lacks them and observe reduced metastasis shows that they are key factors in suppressing lung metastasis.”
Oddly, the rescue failed (that is, metastasis was unchecked) when researchers transfused protective monocytes into mice after cancer cells were injected. This experimental twist provides unanticipated insight into what may be required to halt a metastatic cell: patrolling monocytes had to be in place in the bloodstream before the invaders arrived to keep them from breaching the vessel wall. Showing up after the invasion was simply too late.
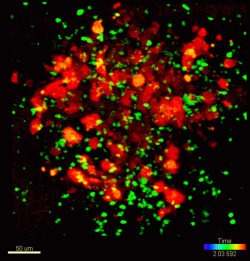
To watch monocyte/tumor cell encounters in real time, the group made videos resembling chase scenes between fluorescently labeled cancer cells and patrolling monocytes in vessels of living mice. In them, glowing green monocytes race after red-tagged cancer cells and apparently physically block them from gaining a foothold in vessel walls, where they could access lung tissue if unimpeded.
But what exactly happened when opponents collided? “We haven’t proven that patrolling monocytes directly kill tumor cells,” cautions Hanna, noting that the patrollers appear to engulf debris from tumor cells but whether they administer the death blow remains unclear. “What is certain is that the patrolling monocytes recruit other immune cells called natural killer cells that are capable of killing tumor cells. Alternatively, these monocytes could scavenge tumor cell debris, which might dampen the inflammatory response.”
This proposed trash-collecting function is not trivial: in fact, there is intense interest in what was once considered membrane garbage thrown off by cancer cells. Researchers now believe that one way in which tumors survive and spread is by lobbing so-called microvesicles at neighbors. Those grenades might contain factors blocking an immune attack or materials needed to construct a tumor blood supply. Thus to undermine a metastatic cell, all a patrolling monocyte may need to do is tidy up after it.
Hanna notes the dire need for treatments for lung metastasis. “Lung metastasis is 85% lethal within 5 years with currently no good treatment options,” he says. “Our study suggests that the next step would be to try to augment existing immunotherapy approaches to prevent tumor metastasis by identifying reagents to increase the number or activity of patrolling monocytes, either as pharmaceuticals or possibly by transferring cells into a patient.”
Hedrick also thinks the new findings could be widely applicable. “In this study we saw effects primarily in lung because there are a lot of these types of monocytes normally present in that tissue,” she says, emphasizing that they did not examine other potential metastatic sites in their mouse model. “Patrolling monocytes may prevent metastasis to other organs as well, a possibility that we will consider in future studies.”
Full citation:
Patrolling Monocytes Control Tumor Metastasis to the Lung. Richard N. Hanna, Caglar Cekic, Duygu Sag, Robert Tacke, Graham D. Thomas, Heba Nowyhed, Erica Shakeri, Nicole Rasquinha, Sara McArdle, Runpei Wu, Esther Peluso, Daniel Metzger, Hiroshi Ichinose, Iftach Shaked, Grzegorz Chodaczek, Subhra K. Biswas, and Catherine C. Hedrick. Science 2015.
Source: La Jolla Institute for Allergy and Immunology
10.11.2015