Article • Methods, quality assurance, commercial providers issues
Molecular testing takes a huge leap
In terms of success in revolutionary cancer treatment, molecular genetic examination procedures have developed immensely over recent years.
Report: Beate Schenk

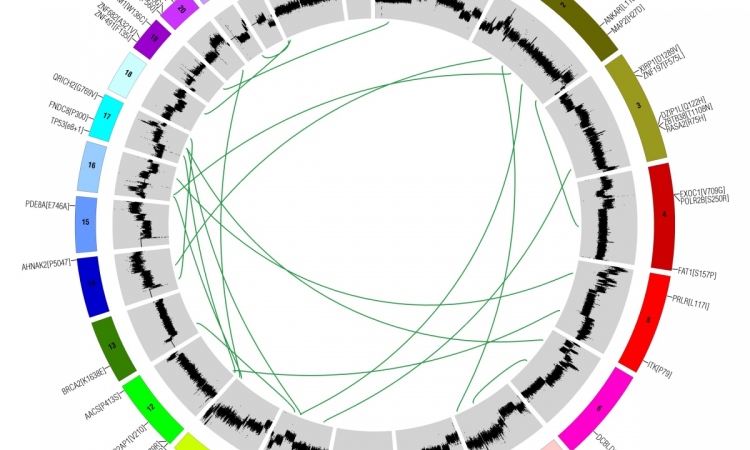
They now range from conventional polymerase chain reactions (PCR) or fluorescence-in-situ hybridisation (FISH) to Next Generation Sequencing (NGS) with analysis of the entire exome or genome (Whole-Exome, WES or Whole-Genome, WGS) and of the transcriptome (RNA-seq) within genetic diagnostics. Here, Professor Wilko Weichert MD, Director of the Institute of General Pathology and Pathological Anatomy at the Technical University of Munich, outlines methods, quality assurance and the role of commercial providers in molecular testing. ‘Fundamentally, conventional in-situ protein analysis is still very important in predictive diagnostics. In part, it has been carried out for decades and is therefore backed by a wide range of data,’ Professor Wilko Weichert points out. ‘Now, along with protein expression analysis, genomics, i.e. sequencing for the detection of mutations, is playing an increasingly important part, often in the shape of multigene analysis, for example. This is currently the backbone of molecular diagnostics.’
Challenges
Weichert emphasises that, despite the increasing differentiation and diversification, molecular diagnostics is no end in itself but an integral part of treatment planning. ‘What use would the best molecular profile of a tumour be if it does not result in any clinical consequences? We have to tailor molecular diagnostics in such a way that it generates information that can be used for therapeutic purposes in the best possible way.’ This is a technological challenge. ‘Complex molecular assays in high-tech patient care originally came from the world of biological research,’ says the pathologist. ‘Their use in medical diagnostics however calls for different qualitative benchmarks, as we must never be wrong.’
In the case of solid tumours, the integration of molecular diagnostics into the entire diagnostic algorithm ensures a choice of the most suitable molecular verification procedure for a problem. According to Weichert, ‘Very different assays can be used for this purpose. Their use also depends on the local environment in which the doctors work and on the expertise available to carry out genetic testing with the respective technologies.’
Diagnostics ‘all inclusive’?
Financing is a particular problem in Germany due to the differentiation between outpatient care and hospital care with all its in-patient flat rates
Wilko Weichert
The financial feasibility of modern molecular diagnostics in healthcare is currently another impediment. ‘Fact: As doctors and scientists, we are repeatedly faced with situations where, from a purely theoretical perspective, we could offer something diagnostically, but for reasons of affordability it’s just not possible for certain clinical situations,’ Weichert explains.
Based on the understanding of scientific medical societies, the costs of molecular analyses, as long as they are specifically for measures of prevention, screening and assurance of diagnosis and/or treatment, must be covered by the cost bearers. In view of fast knowledge growth not only the unified assessment standard (EBM) for out-patient care must be reviewed regularly, but the new examination and treatment procedures (NUB) must also be adapted for in-patient care.
‘Financing is a particular problem in Germany due to the differentiation between outpatient care and hospital care with all its in-patient flat rates. If a new treatment procedure is beneficial for a patient, but not particularly cheap, these flat rates create funding shortfalls. For example, the diagnostic process for critically ill patients is then transferred from in-patient to out-patient care, where the treatment can be funded without putting extra pressure on the flat rate per case system in hospitals. This leads to unnecessary delays in the treatment process.’
QA: the be-all and end-all
Quality assurance in molecular pathology is extremely important. ‘The German national Accreditation Body ensures excellent monitoring of quality standards in pathology,’ Weichert says. ‘Around 100 pathologies have been accredited based on a very strict system; this also includes 40 for molecular pathology. They are basically reviewed on an annual basis. ‘This first cornerstone of quality assurance is complemented by a second one in the shape of its own company: The Quality Initiative Pathology of the German Society for Pathology. ‘This subsidiary of the German Society for Pathology and the Federal Association of German Pathologists carries out numerous ring trials which molecular diagnostic institutes for instance can and must participate in if they want to become accredited.’
At the end of any diagnostic procedure comes an interdisciplinary discussion about patients by a tumour board, to agree on therapy recommendations. ‘We need experts in molecular diagnostics in all regions; pathologists are part of tumour boards and make essential contributions to therapy recommendations with their expertise,’ Weichert points out. In recent years molecular tumour boards with particular expertise in the interpretation of molecular-diagnostic results have developed. These also work together inter-regionally. ‘We will probably have a multistage system in Germany that’s structured into levels of complexity because not all German pathologists will be able to offer the same level of highly complex molecular diagnostics. The procedures are already too complicated for this, and, if you only have a few cases to work on, also too expensive,’ the pathologist believes.
The problem with commercial providers
This can lead to situations where some companies not only offer the diagnostic procedures but also produce the respective medication, and this is not a favourable constellation
Wilko Weichert
The use of commercially orientated molecular diagnostics in treatment is viewed critically by the specialist societies. They fear a development as seen in the USA: ‘Commercial providers have discovered large-scale provision for themselves,’ Weichert observes. In the USA, several private laboratories have been set up by exclusively commercial companies to carry out sequencing. Pathologists send in their tissue samples and receive a written molecular diagnosis. This became possible as comprehensive regional care entailing this type of complex analytics was not possible via the healthcare providers.
These companies are now expanding globally and trying to integrate their business model into the existing German system – a system which, unlike in the USA, is comprehensively structured with high expertise. ‘A cuckoo’s egg, as the technical, molecular-genetic service is taken out of the treatment process and the associated clinical services, such as the discussion of results, and interpretation by tumour boards, must still be provided locally but are not reimbursed,’ Weichert explains.
A further disadvantage is the loss of data sovereignty and the transfer of molecular-genetic patient information. ‘As clinicians, we transfer medical data to a private company paid to work with it. This can lead to situations where some companies not only offer the diagnostic procedures but also produce the respective medication, and this is not a favourable constellation. Health data on the whole is big business.’
Profile:
Wilko Weichert MD is Professor (W3) for Anatomical and Surgical Pathology, and Chairman of the Institute of Pathology, at the Technical University Munich. In his translational tumour research, the professor (author of over 370 original peer reviewed articles), focuses on tissue-based molecular pathology to establish novel predictive, prognostic and diagnostic biomarkers as well as novel therapy targets in solid human tumours. The specific focus is on gastrointestinal, pulmonary, pancreatic plus head and neck carcinomas – he is past-president of the Head and Neck Working Group of the German Society of Pathology.
22.08.2019