© Lumicell
Article • During lumpectomy
Fluorescence-guided surgery identifies breast cancer in margins
Breast surgery is a traumatic experience for a woman, no matter whether breast-conservation surgery (BCS) or a mastectomy. Trauma levels are greatly enhanced, if pathological evaluation findings of an excised breast tumour following a lumpectomy suggest that additional cancer may still be in the margins, and a second surgical procedure is required. A new system with the ability to accurately identify residual tumour in the operating room could reduce re-excision rates, and potentially allow surgeons to excise the tumour with narrower margins.
Report: Cynthia E. Keen
© Lumicell
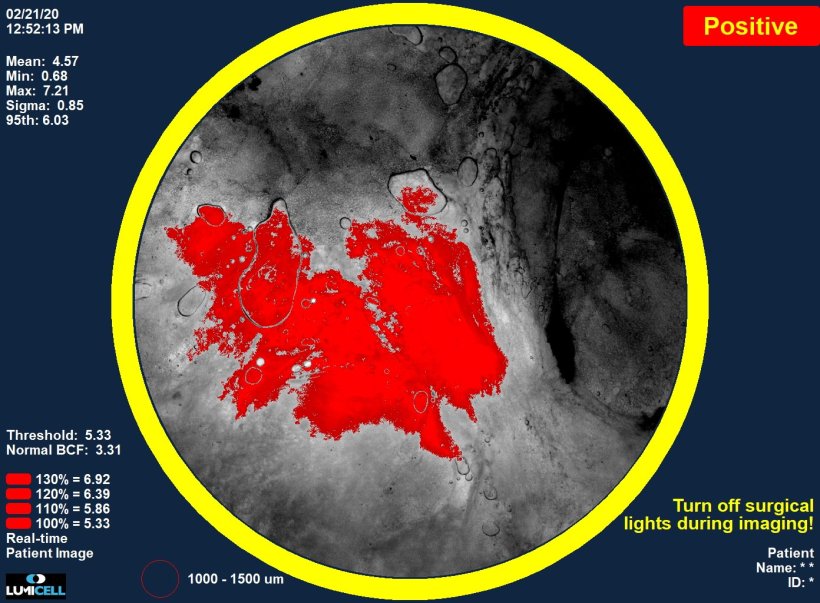
An intracavity visualisation system using fluorescence imaging to identify residual cancer in a tumour bed during a surgical procedure has been successfully tested for performance and safety in a prospective randomized clinical trial involving more than 400 patients undergoing BCS at 14 US cancer treatment centres. Results from the Investigation of Novel Surgical Imaging for Tumor Excision (INSITE) trial revealed that the investigational Lumicell Direct Visualization System identified cancer remaining after a lumpectomy procedure in 7.6% of the participants it imaged. The system, developed by biotechnology company Lumicell, based in Newton, Massachussetts, made 22 of 27 findings from sites deemed negative by standard histopathology assessment. In addition, 14.5% of patients with positive margins had all margins cleared and were spared a second surgery.
The study also evaluated the safety of Lumicell’s investigational optical imaging agent Lumisight (pegulicianine), which becomes fluorescent at residual tumour sites. Pegulicianine had an excellent safety profile, consistent with other imaging agents used in breast conservation surgery, reported INSITE’s lead investigator Barbara Smith, MD, PhD, Professor of Surgery at Harvard Medical School and director of the Breast Program at Massachusetts General Hospital in Boston.
20-40% positive margins
Complete tumour excision during BCS remains a huge challenge because preoperative imaging and current intraoperative examination methods do not accurately identify the microscopic extent of tumours. An estimated 20% to 40% of lumpectomies have positive margins, requiring a second surgery several days later following histopathology analysis. A system with the ability to accurately identify residual tumour in surgical margins in the operating room not only could reduce re-excision rates, but also potentially allow surgeons to excise the tumour with narrower margins.
The pegulicianine fluorescence-guided system (pFGS) includes the injectable activatable fluorescent imaging agent, a handheld probe, and a patient-specific tumour detection algorithm. Images are viewed on a computer screen in the operating room.
All patients were administered 1 mg/kg of the pegulicianine dye two to six hours before surgery. After receiving standard-of-care lumpectomy, 35 patients were randomised to the control arm, and the remaining were assigned to the treatment arm. For the latter group, imaging with the system following tumour excision added approximately 10 minutes to surgical suite time. When intraoperative images demonstrated a red signal indicating pFGS uptake, the surgeons excised additional shave margins in the interior and inferior lateral orientations, until the signal was eliminated from the image displayed on a monitor.
The researchers assessed the diagnostic accuracy of pFGS by comparing each positive or negative image to the histopathological margin assessment at that site. They reported in NEJM Evidence1 and JAMA Surgery2 that pFGS detected tumour tissue that had been left behind by standard lumpectomy in 27 patients. In the 17% who had positive margins after standard surgery, nine of 62 patients had all the margins cleared by pFGS, potentially avoiding a second surgical procedure.
This technology is still in an investigational phase, and additional clinical trials are needed. In addition to breast cancer, its use is being explored for excision of prostate cancer, brain cancers, gastrointestinal cancers, sarcoma, and peritoneal metastases. To date, over 800 patients have been clinically evaluated in seven different cancer types at 18 US medical centres.
References:
05.02.2024





