News • Detection of traumatic brain injury (TBI)
First rapid handheld blood test for concussions receives FDA clearance
Abbott has received 510(k) clearance for the first rapid handheld traumatic brain injury (TBI) blood test, which will help clinicians assess individuals with suspected mild TBIs, including concussions.
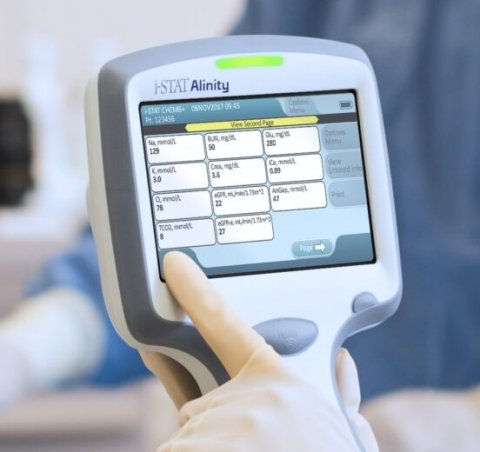
The test will run on Abbott's handheld i-STAT Alinity platform. Tests results are available within 15 minutes after plasma is placed in the test cartridge. TBIs, including concussions, are an alteration in brain function caused by an external force. This test measures specific proteins present in the blood after a TBI. A negative result on this test can be used to rule out the need for a head CT scan, a common tool used to diagnose concussion. For those who test positive, this test result complements CT scans to help clinicians evaluate whether someone has a TBI. "Healthcare providers have been waiting for a blood test for the brain and now we have one," said Beth McQuiston, M.D., medical director for Abbott's diagnostics business. "You can't treat what you don't know and now physicians will be equipped with critical, objective information that will help them provide the best care possible, allowing patients to take steps to recover, prevent reinjury and get back to doing the things they care about most."
The test requires a small blood sample drawn from the arm, from which plasma is extracted with a centrifuge and applied to the test's cartridge. The cartridge is then inserted into the handheld instrument.
Evaluating brain injuries is complex – and research shows that we only catch about half of those who show up to the hospital with a suspected TBI
Geoffrey Manley
Abbott is also working on a whole blood test, which would eliminate the need for separation of plasma and could be used at the patient's side in a healthcare setting. Our vision for the future is that we'd have a 15-minute, portable test that can be used outside the traditional healthcare setting where people experience head injuries and need a quick evaluation, like sporting events.
Abbott has also received FDA breakthrough designation – which speeds assessment of tests through increased FDA interaction – for a TBI test that would run on its Alinity i and Architect core laboratory instruments. This is part of Abbott's strategy to ensure that its tests are available both in the lab and in other settings where people need immediate answers and care.
Nearly 5 million people go to the emergency room for a TBI in the U.S. each year. "Evaluating brain injuries is complex – and research shows that we only catch about half of those who show up to the hospital with a suspected TBI," said Geoffrey Manley, M.D., Ph.D., vice chair of neurological surgery at the University of California, San Francisco. "And beyond those who go to the hospital for a suspected TBI, many more never do. A test like this could encourage more people to get tested after a head trauma, which is important, because not receiving a diagnosis can be dangerous and may prevent people from taking the necessary steps to recover safely."
Survivors of TBI may experience impairment of memory, movement, sensation (e.g., vision and hearing), and emotional functioning (e.g., personality changes, psychological symptoms). Effects of TBI can last anywhere from a few days post-injury or may be permanent. People who sustain a TBI are more likely to have another one – similarly to how a sprained ankle or torn ligament is more susceptible to future injury. These effects are worsened by misdiagnosis or lack of diagnosis. Abbott's blood test will give healthcare professionals an objective tool for evaluating people suspected of having an injury to the brain.
Source: Abbott
13.01.2021