News • New report
Advanced development of primary pancreatic organoid tumor models for high-throughput phenotypic drug screening
A multidisciplinary team of scientists share recent advancements in innovative in-vitro cancer biology methods for screening drug-like molecules in cancer tissue relevant models in a new report published online ahead-of-print at SLAS Discovery.
A multidisciplinary team of scientists share recent advancements in innovative in-vitro cancer biology methods for screening drug-like molecules in cancer tissue relevant models in a new report published online ahead-of-print at SLAS Discovery. Entitled Advanced Development of Primary Pancreatic Organoid Tumor Models for High-Throughput Phenotypic Drug Screening, the report can be accessed for free.
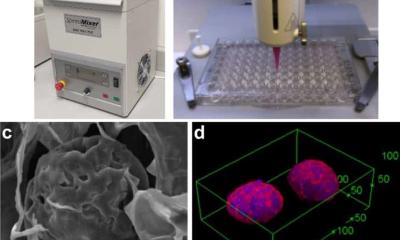
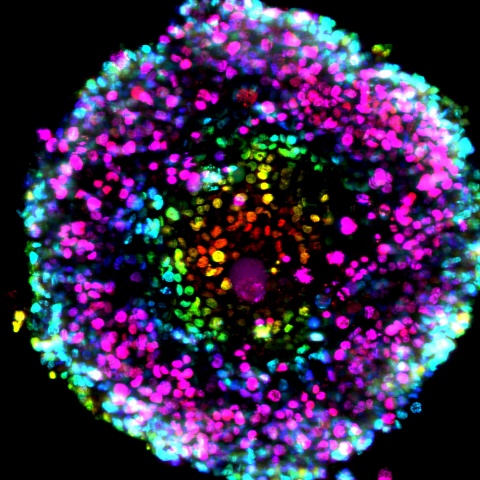
The authors -- Senior Scientific Directors Timothy Spicer and Louis Scampavia and Post-Doctoral Associate Shurong Hou at Scripps Florida in collaboration with Cold Spring Harbor Laboratory, Greiner Bio-One, Nano3D Biosciences, Inc., University of Texas Health Science Center at Houston, and the Dana-Farber Cancer Institute -- illustrate how a magnetic nanoparticle assembly approach is used to increase throughput dramatically while reducing costs. This technology combines specialized high-density microtiter plates formulated with an ultra-low attachment surface along with gold nanoparticles (nanoshuttles), which are used to label cancer cells in-vitro. Once labeled, a magnetic driver quickly pulls the cells into a 3D spheroid or organoid structure. This 3D structure is retained and drug–like molecules can then be added, affording the ability to ascertain their efficacy.
With the advent of cost-effective and high-throughput 3D tissue culture, the importance of developing this technology using patient-derived cancer cells is to ensure a more disease and physiologically relevant point of comparison to 2D monolayer testing, thereby validating the hypothesis of 3D relevance as a predictor of possible patient outcomes. This allows researchers to step closer to identifying patient-specific therapies and points in the direction of rapid 3D testing of patient-derived cancer cells against FDA-approved drugs, which may be both affordable and amenable for a precision medicine approach to provide timely and critical feedback to physicians.
Source: SLAS (Society for Laboratory Automation and Screening)
20.04.2018