Article • Therapy monitoring
Liquid biopsy versus radiomics – the race is on
The development of new procedures to monitor cancer treatments is gathering momentum. One such innovation is liquid biopsy.
Interview: Daniela Zimmermann
This new lab technique allows non-invasive identification, characterisation and monitoring of circulating tumour DNA. Thus, liquid biopsy can potentially revolutionise oncological diagnostics – and put a spoke in the wheel of radiology. High time to act, says Professor Dr Jens Ricke, Chair of Radiology at Ludwig Maximilian University (LMU), Munich, and Director of the Clinic and Polyclinic of Radiology at LMU Hospital. He is confident that radiology has several aces up its sleeve – one is radiomics.
Why is liquid biopsy such a success?

As soon as liquid biopsy has identified circulating tumour DNA, it’s also possible to identify gene mutations that might influence the therapy. This effect can be seen in the personalised therapy of colorectal cancer: an RAS or RAF mutation of the metastasising colorectal tumour impacts systemic therapy. Liquid biopsy can indeed detect this mutation in circulating tumour DNA.
What does that mean for imaging?
There are better parameters, which have not yet been clinically validated and are thus not accepted as follow-up variables
Jens Ricke
That’s exactly the sore point. Methods such as liquid biopsy are mere lab procedures. They have only recently been introduced and are still under development. This phase, no doubt, will continue for a while. Nevertheless, we radiologists have to get a move on today if we want to actively shape the future of oncological imaging. If liquid biopsy keeps its promise, it may well replace the tight imaging follow-up in oncology. Obviously, that won’t mean that radiologists will be out of work. But, we do have to think about our future now and, above all, we need to tap the full potential of radiology. For a long time, we have applied RECIST (Response Evaluation Criteria in Solid Tumours) criteria and accepted their imprecisions. However, we do need unambiguous standards. The current procedure relies on mere volume measurement: tumour growth or regression, however minute, is evaluated. This, however, does not allow a reliable prediction of therapy response. There are better parameters, which have not yet been clinically validated and are thus not accepted as follow-up variables.
Enter radiomics: future developments in radiomics may well yield suitable criteria to gauge therapy response in oncology. However, as yet there is no proof that the parameters really predict patient survival linked to therapy response. Current data are incomplete and their analysis and successful transformation into criteria will take years. Therefore, we must hurry up. The competition is on the ball. This is why we should invest more resources in radiomics. I’m sure we do have the means and the possibilities to create useful alternatives.
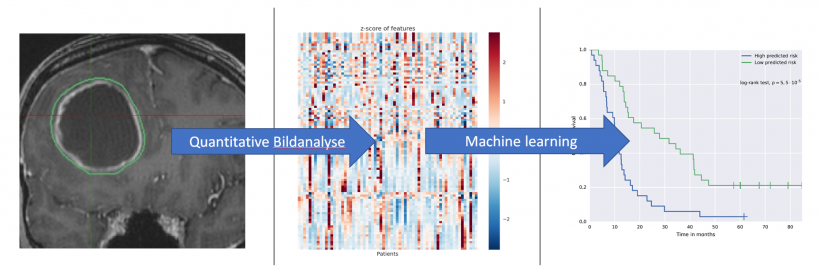
What exactly needs to be done to shape this future?
Above all, we need validation studies. Firstly, imaging methods need to be established, which might encompass entirely new forms of analyses. The idea underlying radiomics is to cull more information from image data than meet the human eye. This involves complex statistical analyses, what’s usually subsumed under the buzzword Big Data: computational drudgery. Every large data set has to be analysed over and over until patterns with predictive value regarding therapy response – or the lack thereof – become visible. MRI has already offered promising techniques. But this is just the beginning. The more methods are being developed, the more important is the validation of those methods. Prospective clinical studies that define and show innovative imaging endpoints for therapy response are indispensable. Today, FDA and EMA require clinical trials for regulatory approval of a pharmaceutical to be based on the RECIST criteria. However, we also have to think about the validation of potentially new imaging endpoints.
How quickly can results be produced?
The validation of such procedures is time-consuming. This is another reason why the projects have to be launched as soon as possible. Validation is always tied to a specific endpoint of the study as such, which might also include long-term survival studies. However, not only studies themselves can take years, the preparation also takes a lot of time.
Is there political or industry support for these methods?
Such studies are eligible for public funding; but even more interesting is the interest of the pharmaceutical industry. Validation would require clinical trials that include new imaging standards. These studies are rarely publicly funded. By the way, within the pharmaceutical industry the awareness that new imaging endpoints need to be defined has markedly increased with the success of immunotherapies. This is because, in the initial phase of immunotherapy, a response in the traditional sense cannot be determined. Quite to the contrary: tumours tend to grow despite an initially positive response. This is caused by tumour infiltrating lymphocytes, which in fact trigger an inflammation. This is an accompanying effect of immunotherapy. These lymphocytes cause pseudoprogression of the tumour, which a conventionally trained radiologist or oncologist might interpret as tumour growth – an undesired outcome – and discontinue the therapy. However, in many cases, initial tumour growth during immunotherapy turns into tumour regression – the therapy should have continued rather than stopped.
To account for these new insights RECIST criteria were expanded: the new so-called iRECIST criteria recommend a different evaluation of immunotherapy response. If tumour growth is recorded, further controls are performed in short intervals to determine whether this is a bona fide progression or whether the tumour indeed shrinks after a while. The oncology community doesn’t yet appear to be entirely convinced of the progression/regression dynamics; that’s why, in clinical practice, we see immunotherapy medication being discontinued sooner than in the trials. This is obviously to the detriment of the patient, because successful medication is no longer administered. At the same time it is to the detriment of the pharmaceutical companies, because their turnover decreases. This is why the pharmaceutical industry, patients, oncologists and radiologist have a common interest in the development of new imaging endpoints. We should pick up the ball and run.
Profile:
Professor Jens Ricke qualified in radiology at Charité, Berlin, Germany and, from 2004 to 2006, was professor of interventional radiology at the Department of Radiotherapy there. From 2006 to 2017 he was tenured professor of radiology at Otto von Guericke University Magdeburg, and Director of the Department of Radiology and Nuclear Medicine at Magdeburg University Hospital in Germany. In June 2017, he joined Ludwig Maximilian University, Munich, Germany, where he is Chair of Radiology and Director of the Clinic and Policlinic of Radiology.
27.02.2018