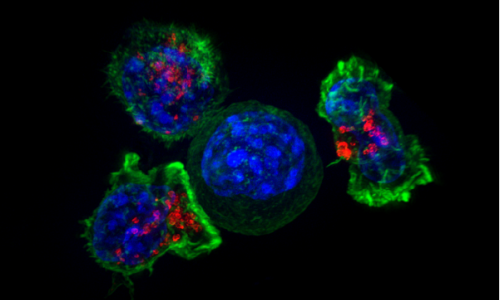
Credit: Ariel Ruiz i Altaba
News • Cancer research
Origins of metastasis unraveled
For a long time, the origin of metastasis remained obscure. Now, scientists at the University of Geneva (UNIGE) have discovered some of the mechanisms these cells arise.
Metastatic cells occur in many forms of cancer. They originate in primary tumors and then break away and migrate. They travel through the tissues surrounding them, through blood vessels or lymphatic channels. Along the way, they may attach to one or more organs—such as the lungs, brain, bones or liver—and form new tumors also called metastases. This spread reduces patients' chances of recovery.
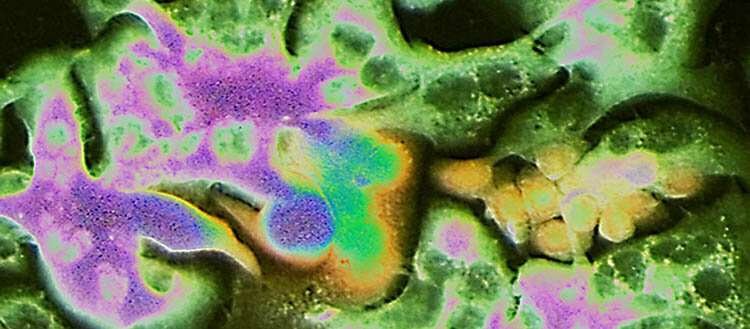
Scientists at the University of Geneva (UNIGE) have discovered some of the mechanisms by which these cells arise. This is due to cells that have narrowly escaped cell death (apoptosis) following a chemotherapeutic treatment. Those cells reprogram themselves to acquire metastatic skills. Thanks to this study, these cells—called PAME by the researchers—now appear as new therapeutic targets.
Previous studies have identified metastatic cells during migration. It is also known that certain treatments can induce them. However, the precise mechanisms of their development remain a mystery. "We don't know why, at a given moment, certain cells separate from the primary tumor," explains Ariel Ruiz i Altaba, a Full Professor in the Department of Genetic Medicine and Development at the UNIGE Faculty of Medicine. "The phenomenon is difficult to analyze because, before they migrate, there is nothing to distinguish future metastatic cells, or pro-metastatic cells, from other cells within the tumor."
Cells that should have died
Professor Ruiz i Altaba's team—composed of two postdocs for this study, Arwen Conod (first author) and Marianna Silvano—has now provided some answers. Thanks to recent research, these UNIGE scientists have discovered that the experience of imminent death within the primary tumor pushes certain cells to acquire pro-metastatic states. This near-death experience occurs in particular in the context of certain treatments aimed at depriving cancer cells of energy or oxygen. The team observed that these cells, which should have died, reprogram themselves and then present a high metastatic risk. These cells are called PAME for "post-apoptotic pro-metastatic cells."
A storm of cytokines
To reach these conclusions, the UNIGE team used tumor samples taken from two colon cancer patients. Tumor cells from these samples were then transplanted into mice, where they grew and formed new tumors. These cells were subjected to an imminent death experience causing endoplasmic reticulum stress similar to that caused by certain chemotherapeutic drugs. This allowed the development of PAME cells.
The scientists also discovered that PAMEs trigger a storm of cytokines—proteins and other factors that ensure cell-to-cell communication—inducing adjacent cells to become PAME-induced migratory cells (PIMS). These PIMs then associate with PAMEs and help them migrate to form metastases.
The present results open up promising new prospects for therapeutic management, including the prevention of the development of pro-metastatic fields generated by certain treatments.
"Currently, one of the main criteria when defining a treatment is tumor shrinkage. Thanks to our study, PAME cells now appear as potential therapeutic and metastasis prevention targets to be taken into account," concludes Professor Ruiz i Altaba.
The study was published in the journal Cell Reports.
Source: University of Geneva
10.03.2022