Image source: VHIO
News • Research on RAD51 biomarker
Next-generation sequencing for personalizing prostate cancer treatment
Results of a study led by the Vall d'Hebron Institute of Oncology (VHIO) support the feasibility of using RAD51 testing to complement next-generation sequencing (NGS) for precise patient stratification and treatment selection in metastatic prostate cancer (mPC).
The researchers' insights were published in Cell Reports Medicine.
Different genomic alterations in DNA damage repair (DDR) pathways occur in 20% to 25% of advanced prostate cancer, including homologous recombination repair (HRR) gene alterations. Identifying the molecular characteristics and targetable mutations of each tumor has become an integral part of metastatic prostate cancer care. “The association of homologous recombination repair defects with response to treatment with PARP inhibitors marked the first successful application of precision medicine in patients with prostate cancer,” said Joaquin Mateo, a medical oncologist at the Vall d’Hebron University Hospital, co-leader of VHIO’s Prostate Cancer Group, and co-corresponding author of this study. “However, several challenges of NGS testing still impede the widespread implementation of precision medicine in routine metastatic prostate cancer care,” he added.
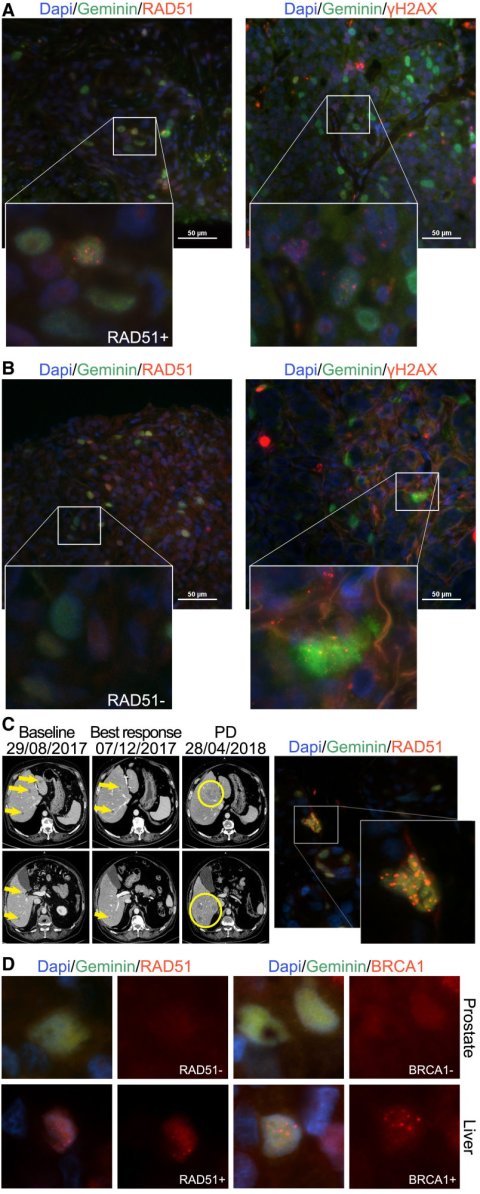
Image source: Arce-Gallego S, Morgado PC, Delgado-Serrano L et al., Cell Reports Medicine 2025 (CC BY-NC-ND 4.0)
“Refining cancer biomarker strategies and identifying new tumor markers that complement current NGS methods could facilitate a more precise patient stratification and treatment selection process” said Violeta Serra, Head of VHIO’s Experimental Therapeutics Group and co-corresponding author of this present article.
RAD51 is a protein involved in HRR alterations. Developed in-house by Serra’s laboratory, the RAD51 assay is based on the detection of this protein as a functional biomarker to potentially enhance the stratification of patients with cancer associated with deficiency in this DNA damage repair (DDR) pathway. Functional assays that can detect dynamic changes in HRR capacity could also support patient stratification strategies and help prioritize NGS testing when resources are limited.
For this study, the investigators performed a comprehensive analysis of homologous recombination repair status through parallel evaluation using NGS and the RAD51 test in 219 primary or metastatic biopsies from 187 patients with advanced prostate cancer. Genomic analysis revealed that the most frequently altered genes included TP53, PTEN, AR, MYC, BRCA2, ATM y BRCA1, highlighting the complex genomic landscape of mCRC. RAD51 immunofluorescence showed that 21% of evaluable samples had a RAD51-low score, indicating HRR deficiency, and a high sensitivity and specificity for identifying tumors with BRCA1/2 alterations. Patients with RAD51-low scores experienced longer progression free survival (PFS) on treatment with PARP inhibitors or platinum chemotherapy.
“Our results demonstrate the feasibility of using the RAD51 biomarker to identify patients with homologous recombination repair-defective prostate cancer and who may therefore be sensitive to treatment with PARP inhibitors,” observed Violeta Serra. “This biomarker could complement NGS in clinical practice, particularly in cases with limited tissue availability where sequencing may not be possible,” concluded Mateo.
Source: Vall d'Hebron Institute of Oncology
09.02.2025