News • Endoscopy RFA
New tool opens doors for pancreatic cancer treatment
A significantly more effective, minimally invasive treatment for pancreatic tumors may be on the horizon, thanks to a new endoscopy tool created in the Penn State Department of Mechanical Engineering.
On average, only about 20 percent of pancreatic cancer patients are eligible for a surgical removal of the tumor, which is currently the most-effective treatment option. The location of the pancreas in the abdomen and the difficulty to detect the disease make it one of the most difficult to treat. To change that prognosis, Brad Hanks, a doctoral student studying mechanical engineering, created a new type of electrode to be used in endoscopic radiofrequency ablation (RFA) procedures. His work in the Engineering Design and Optimization Group (EDOG) has been theorized to effectively neutralize 55 percent more of an abdominal tumor using RFA methods.
Without a surgical tool to match the shape of the tumor, the effectiveness of the treatment can be severely limited
Brad Hanks
A minimally invasive procedure, RFA is conducted by inserting an electrode into the abdomen and administering high-frequency energy that heats the tumor and as a result, neutralizes the cancer cells. While the RFA treatment itself is well-established and effective, the current endoscopic tools that exist to perform this procedure are “one size fits all”, rather than customized to each person’s tumor. A standard RFA electrode produces an elliptical ablation zone, which most tumors are approximately spherical. Since the RFA treatment zone doesn’t usually match the shape of the tumor, the untreated remnants can continue the spread of the disease. “It’s like trying to fit a square peg in a round hole,” Hanks said. “Without a surgical tool to match the shape of the tumor, the effectiveness of the treatment can be severely limited.”
However, by harnessing finite element analysis and evolutionary algorithms, he designed an electrode that deploys once it is within the abdomen, spreading electrode “fingers” that produce an ablation zone that is better matched to the specific tumor’s shape. “Because each cancer patient is unique, I believe the tools we use to treat them should be as well,” he said. Though the electrode itself may not able to completely eradicate the tumor, the method also provides additional benefits to a patients’ on-going treatment, an idea from Hank’s collaborator on the project, Matthew Moyer, a physician at the Penn State Hershey Medical Center.
3D printing patient-specific electrodes
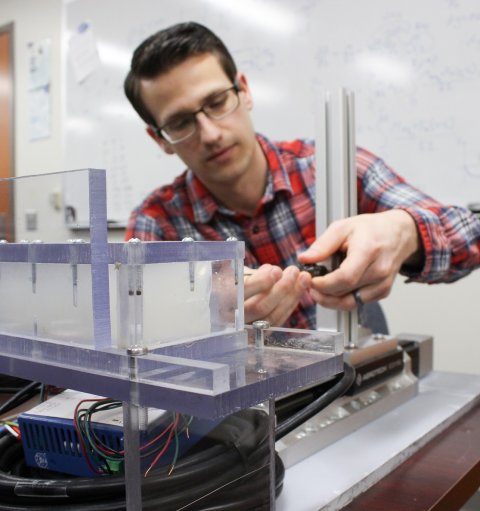
Currently, gold beads called fiducials are often inserted in another procedure to provide guidance for radiation treatments. Marking the edges of the tumor through X-rays, the fiducials provide a clearer outline for targeting and eradicating the tumor. The custom electrode, designed to detach and stay in the tumor, can provide a similar purpose. With one less procedure, Hanks said, “Using my electrodes, which are able to do the same thing, it’s a simplified process to have this electrode guide the way for radiologists.” In addition, with further exploration, Hanks hopes he can 3D print patient-specific electrodes to ensure an even more targeted and personalized treatment. He said, “It adds another really interesting component to this work, it could lead to an even more custom treatment that is less expensive for the patient.”
While the initial focus of the project was on treating pancreatic cancer tumors due to the low rate of patients eligible for surgery and its status as the 4th most deadly cancer in the United States, the custom electrode treatment can be adapted for other abdominal cancers, including tumors found in the lungs and stomach.
Recommended article
News • Biomarker validation
Plodding toward a pancreatic cancer screening test
Pancreatic cancer is one of the most deadly types of malignancies, with a 5-year survival rate after late diagnosis of only about 5%. The majority of patients—about 80%—receive their diagnosis too late for surgery. The disease spreads quickly and resists chemotherapy. In short, there is an urgent need for diagnostic tools to identify this cancer in its earliest stages.
The product is currently in development with Actuated Medical, Inc., a company based in Bellefonte, Pennsylvania that develops next-generation FDA-compliant medical devices. Hanks continues to conduct some testing of the product in the EDOG lab and mentor undergraduate students participating in the project, but has largely passed the baton to Kevin A. Snook, the medical transducer design leader and senior research engineer at Actuated Medical, to bring the device to market.
“We help support the company with simulations and experiments and having a partner in industry has been a great experience,” Hanks said. “I’m hopeful that patients will one day be able to benefit.” He added, “The day we are able provide truly personalized care for these cancers is not so far away.”
Source: Penn State College of Engineering
29.01.2019