Video • Exposing the enemy
New algorithm detects even the smallest cancer metastases
Teams at Helmholtz Zentrum München, LMU Munich and the Technical University of Munich (TUM) have developed a new algorithm that enables automated detection of metastases at the level of single disseminated cancer cells in whole mice.
© Helmholtz Zentrum München
The researchers published their findings in the journal Cell.
Cancer is one of the leading causes of death worldwide. More than 90% of cancer patients die of distal metastases rather than as a direct result of the primary tumor. Cancer metastases usually develop from single disseminated cancer cells, which evade the body’s immune surveillance system. Up to now, comprehensive detection of these cells within the entire body has not been possible, owing to the limited resolution of imaging techniques such as bioluminescence and MRI. This has resulted in a relative lack of knowledge of the specific dissemination mechanisms of diverse cancer types, which is a prerequisite for effective therapy. It has also hampered efforts to assess the efficacy of new drug candidates for tumor therapy.
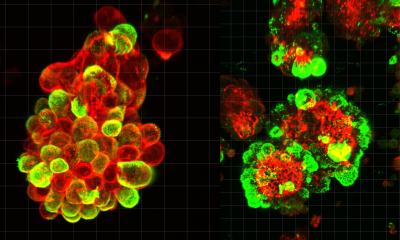
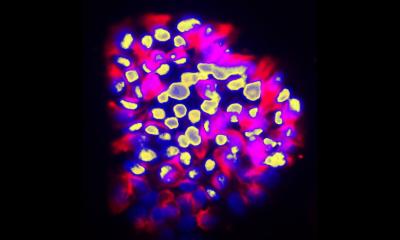
In order to develop new techniques to overcome these hurdles, the team led by Dr. Ali Ertürk, Director of the Institute for Tissue Engineering and Regenerative Medicine at Helmholtz Zentrum München, had previously developed vDISCO – a method of tissue clearing and fixation which transforms mouse bodies into a transparent state allowing the imaging of single cells. Using laser-scanning microscopes, the researchers were able to detect the smallest metastases down to individual cancer cells in cleared the tissue of the mouse bodies.
However, manually analyzing such high-resolution imaging data would be a very time-consuming process. Given the limited reliability and processing speed of currently available algorithms for this kind of data analysis, the teams have developed a novel deep-learning based algorithm called DeepMACT. The researchers have now been able to detect and analyze cancer metastases and map the distribution of therapeutic antibodies in vDISCO preparations automatically. The DeepMACT algorithm matched the performance of human experts in detecting the metastases – but did so more than 300 times faster. “With a few clicks only, DeepMACT can do the manual detection work of months in less than an hour. We are now able to conduct high-throughput metastasis analysis down to single disseminated tumor cells as a daily routine”, says Oliver Schoppe, co-first-author of the study and Ph.D. student in the group of Prof. Dr. Bjoern Menze at TranslaTUM, the Center for Translational Cancer Research at TUM.
Recommended article

Article • Cancer of unknown primary
CUP: in search for the smoking gun
Cancer of unknown primary (CUP) can send radiologists on a frustrating scavenger hunt: metastases were detected but the primary cancer is nowhere to be seen. Professor Alwin Krämer, Head of the Clinical Cooperation Unit Molecular Haematology/Oncology at University Hospital Heidelberg and the German Cancer Research Center, explains strategies for dealing with CUP.
In order to develop more effective cancer therapies, it is critical to understand the metastatic mechanisms in diverse cancer types and to develop tumor-specific drugs that are capable to stop the metastatic process
Ali Ertürk
Using DeepMACT, the researchers have gained new insights into the unique metastatic profiles of different tumor models. Characterization of the dissemination patterns of diverse cancer types could enable tailored drug targeting for different metastatic cancers. By analyzing the progression of breast-cancer metastases in mice, DeepMACT has uncovered a substantial increase in small metastases throughout the mouse body over time. “None of these features could be detected by conventional bioluminescence imaging before. DeepMACT is the first method to enable the quantitative analysis of metastatic process at a full-body scale”, adds Dr. Chenchen Pan, a postdoctoral fellow at Helmholtz Zentrum München and also joint first author of the study. “Our method also allows us to analyze the targeting of tumor antibody therapies in more detail.”
With DeepMACT, the researchers now have a tool with which to assess the targeting of clinical cancer therapies that employ tumor-specific monoclonal antibodies. As a representative example, they have used DeepMACT to quantify the efficacy of a therapeutic antibody named 6A10, which had been shown to reduce tumor growth. The results demonstrated that 6A10 can miss up to 23% of the metastases in the bodies of affected mice. This underlines the importance of the analysis of targeting efficacy at the level of single metastases for the development of novel tumor drugs. The method can potentially also track the distribution of small-molecule drugs when they are conjugated to fluorescent dyes.
Taken together, these results show that DeepMACT not only provides a powerful method for the comprehensive analysis of cancer metastases, but also provides a sensitive tool for therapeutic drug assessment in pre-clinical studies. “The battle against cancer has been underway for decades and there is still a long way to go before we can finally defeat the disease. In order to develop more effective cancer therapies, it is critical to understand the metastatic mechanisms in diverse cancer types and to develop tumor-specific drugs that are capable to stop the metastatic process,” explains Ertürk.
DeepMACT is publicly available and can be easily adopted in other laboratories focusing on diverse tumor models and treatment options. “Today, the success rate of clinical trials in oncology is around 5%. We believe that the DeepMACT technology can substantially improve the drug development process in preclinical research. Thus, could help finding much more powerful drug candidates for clinical trials and hopefully help to save many lives”.
Source: Helmholtz Zentrum München
13.12.2019