News • Radiopharmaceutical tracers
Diagnosing and treating tumors with radioactive metal complexes
A team under the direction of chemist Prof. Dr Peter Comba is investigating radioactive metal complexes for use in the diagnosis and treatment of tumors.
In their recent studies at Heidelberg University's Institute of Inorganic Chemistry, the researchers demonstrated that developing radiopharmaceutical tracers based on indium and actinium shows great promise for new radiopharmaceuticals. The results from this basic research will be used in further studies with a view toward possible applications.
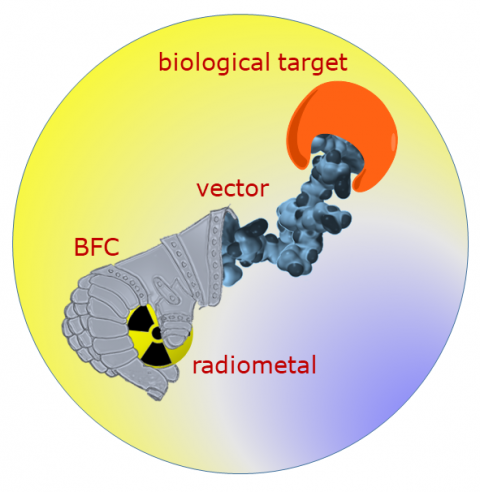
The radiopharmaceutical tracer concept uses a biological vector to locate diseased tissue in the organism. The vector, such as a peptide or an antibody, is marked with a radioactive element and administered to the patient. This radiative unit accumulates at its target, and depending on the element's decay process, the radiation can render the tumour cells visible or destroy them. “One important advantage of this method is that it can be used to find individual cells and thus allow treatment of very small tumours,” explains Prof. Comba. The radioactivity in these drugs is so strong that only very small concentrations are needed to visualise or destroy tumours. Picomolar to nanomolar solutions are used. The concentration of radioactive atoms in such a solution is about one million times smaller than that of sodium ions in the blood.
Because the radioactivity gets distributed throughout the entire body, it would be disastrous for sharp images or the selective destruction of tumor cells
Peter Comba
According to Prof. Comba, there are many reasons for labelling biological vectors with radioactive metal ions. There is a wide range of available elements and isotopes with ideal half-life times, decay processes and energy for diverse applications. The metal ions are bound to organic molecules called bifunctional chelators (BFCs), which in turn are attached to the tumor-seeking biological vectors.
Speed and efficiency are important in marking the tracers with radioactive metal ions. It must also be done under physiological conditions so that the biological vectors remain undamaged. It is additionally critical that the radioactive atom is strongly bound to the BFC. “Under no circumstances should it get lost on the way to the tumor cell,” explains Prof. Comba. “Because the radioactivity gets distributed throughout the entire body, it would be disastrous for sharp images or the selective destruction of tumor cells.”
Rapid labelling, an extremely small concentration and high stability are conditions that are very difficult to achieve simultaneously. In developing the special tracer molecules, the Heidelberg researchers are focused on BFCs, whose structure is similar to the extremely stable diamond geometry. Over the last several years, Prof. Comba's team had already demonstrated these BFCs to be an extremely promising platform for developing radiopharmaceutical tracers with copper ions – in this case copper-64.
Further work sought to extend the application spectrum to other radiometals important in nuclear medicine. The actinium-225 isotope is of particular interest; until now, no BFC has proved potent enough. For her doctoral dissertation, Dr Katharina Rück of Prof. Comba's team concentrated on synthesising new types of bifunctional chelators for radioactive metal ions such as actinium-225. These new BFCs were studied extensively in collaboration with colleagues in Canada, who also conducted the radiochemical studies.
The results are quite promising using indium-111 for diagnosis and actinium-225 for therapy, which means that the same tracer but with different metal ions can be used for both diagnosis and therapy. The new BFC will now be coupled to biological vectors and tested in animals.
Source: Heidelberg University'
13.09.2017