Image credit: Children's Medical Research Institute
News • Surprising insights
Research explains how tumor cells die after radiotherapy
Scientists at Children’s Medical Research Institute (CMRI) have solved a big mystery in cancer research – why cells die in different ways following radiotherapy.
This surprising finding opens up new opportunities to improve treatment and increase cure rates. The findings were published in Nature Cell Biology by first author Dr Radoslaw Szmyd of CMRI’s Genome Integrity Unit, which is led by Professor Tony Cesare.
Radiation therapy (also called radiotherapy) is a critically important type of cancer treatment. Scientists have struggled for decades to understand why radiation therapy kills cells from the same tumour in different ways. This is important because some forms of cell death are unnoticed by the immune system, while others trigger an immune response that kills other cancer cells. Unleashing the patient’s immune system to kill cancer cells and clear tumours is a major goal of cancer treatment.
Live imaging showed us the full complexity of outcomes following radiation therapy, allowing us to tease out exactly why this occurred
Tony Cesare
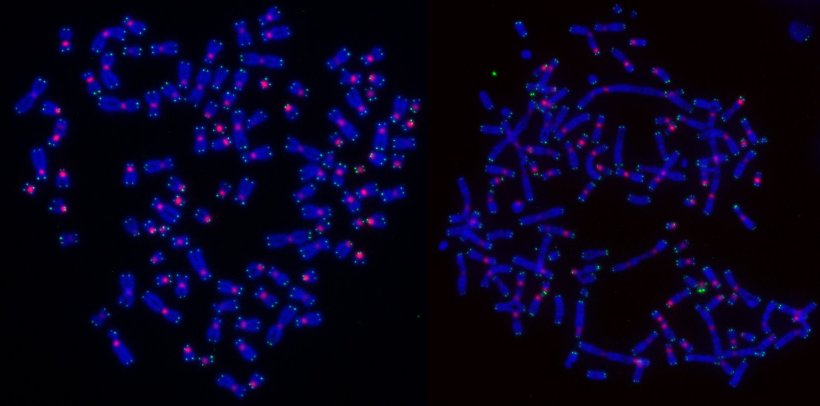
“The surprising result of our research is that DNA repair, which normally protects healthy cells, determines how cancer cells die following radiotherapy,” said Prof Cesare. “The DNA inside our cells is constantly experiencing damage, and DNA repair is happening all the time to fix that damage and keep our cells healthy. Now, however, it seems these repair processes can recognise when overwhelming damage has occurred (e.g., from radiotherapy), and instruct a cancer cell how to die. When DNA damaged by radiation therapy was repaired by a method called homologous recombination cancer cells died during the process of reproducing – a process called cell division or mitosis. Critically, death during cell division goes unnoticed by the immune system, so it won’t activate an immune response. This is not what we want. However, cells that dealt with the radiation-damaged DNA through other DNA repair methods survived the cell division process but did so by releasing byproducts of DNA repair into the cell. To the cell, these repair byproducts look like a viral or bacterial infection. This causes the cancer cell to die in a manner that alerts the immune system. Which is what we do want.”
The team showed that blocking homologous recombination changed the way the cancer cells died – i.e., they now died in a manner that evoked a strong immune response. The team also found that cancer cells that have mutations in BRCA2 – a gene that is very important for breast cancer and which is necessary for homologous recombination – do not die in mitosis following radiotherapy. In addition to solving a major scientific puzzle, these discoveries will make it possible to use drugs that block homologous recombination to force cancer cells treated with radiotherapy to die in a manner that alerts the immune system to the existence of a cancer, (which the immune system had not previously noticed), signalling that the cancer needs to be destroyed. Prof Cesare credits these breakthroughs to live cell microscope technology that enabled his team to follow irradiated cells for a week following radiation therapy. “Live imaging showed us the full complexity of outcomes following radiation therapy, allowing us to tease out exactly why this occurred.”
Co- project lead, A/Prof Harriet Gee, a radiation oncologist from the Western Sydney Local Health District Radiation Oncology Network, said these findings answer a clinical question that has puzzled the field for 30 years. “We found that the manner in which tumour cells die after radiotherapy depends on the engagement of specific DNA repair pathways, particularly when radiation is given at very high, focussed doses. This opens up new opportunities to enhance radiation efficacy through combination with other therapies, particularly immunotherapy, to increase cancer cures.’’
Prof Cesare said Dr Szmyd worked for six years on this “incredibly difficult nut to crack’’ and “The perseverance required for a project of this scope is a testament to Radek and the team. Everyone is aware of patients battling cancer. Discovering something like this that has the potential to make a big difference to people’s lives is very rewarding.’’
Authors on the paper include CMRI researchers Sienna Casolin, Lucy French, Dr Anna Gonzalez-Manjon, Dr Melanie Walter, Lea Cavalli, Scott Page, Prof Hilda Pickett, Dr Chrisopher Nelson, and Dr Andrew Dhawan from the Neurological Institute at the Cleveland Clinic in the US and A/Prof Eric Hau from the Westmead Clinical School at the University of Sydney.
Source: Children's Medical Research Institute
13.01.2025