Source: Pexels/rawpixel.com
News • Fake pills
'Decoy' antibiotics dupe bacteria’s defences
Imperial medical students have helped to devise a new type of ‘decoy’ drug to tackle infections that are resistant to antibiotics.
In lab tests on bacterial cultures, the new drug successfully killed a strain of drug-resistant bacteria. It works by delivering two antibiotics, one of which is effectively hidden. When the bacteria fight against the first ‘decoy’ antibiotic, this action opens up the drug, triggering the second antibiotic into action. This enables the second antibiotic to be delivered in a targeted way, only being released where it encounters drug-resistant bacteria. The findings could help prolong the life of existing antibiotics by slowing the rate at which bacteria become resistant to them.
Antibiotic resistance is a growing problem worldwide, with microbes evolving ways to avoid being killed by a range of medications. It is impacting the fight against TB, HIV and malaria, as well as threatening the success of major surgery and cancer chemotherapy.
Recommended article

Article • Antimicrobial resistance
What to do when antibiotics are taken like candy?
The need to do more to combat resistance in Germany was keenly emphasised by delegates at the VDGH (The German Diagnostics Industry Association) event held in Berlin this May. Only the answer, or at least the common will, is missing to bring this threat under control via a concerted concept.
Now, researchers at Imperial College London have designed a new way to deliver antibiotics that only targets and kills bacteria that have evolved a mechanism of resistance to frontline antibiotics. Any remaining infection caused by non-resistant bacteria can be cleared using normal penicillin. Their results are published in the Journal of Medicinal Chemistry.
Setting a trap
No matter how good bacteria are at evolving resistance to antibiotics they can never ‘think ahead’
Thomas Webb
A wide range of bacteria, including E. coli, have evolved a special enzyme that can cleave (split) frontline antibiotics known as cephalosporins, rendering them useless. To get around this, the team attached a cephalosporin molecule to a secondary antibiotic called ciprofloxacin. When resistant bacteria encounter the combined drug they cleave the cephalosporin, setting the ciprofloxacin free to kill the bacteria. Ciprofloxacin is rarely used as a primary treatment for infections because it can have severe side effects. However, since it is only activated when the cephalosporin is cleaved, the levels in the bloodstream are much lower and will hopefully lead to fewer side effects. Co-author Dr Thomas Webb said: “No matter how good bacteria are at evolving resistance to antibiotics they can never ‘think ahead’, and this is why we believe setting a trap for them in this way may be so effective.”
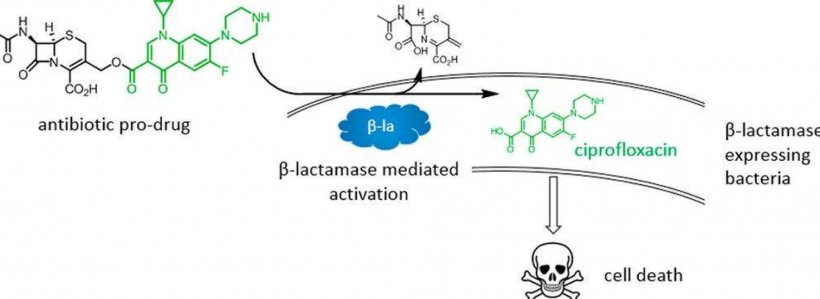
© Imperial College London
Fighting resistance
Antibiotic resistance often emerges because drugs are used indiscriminately against any infection, without knowing its specific cause. This method targets the weakest bacteria, leaving the strongest bacteria to survive and pass on their genes for resistance. However, the new drug is more specific, targeting only already-resistant bacteria. Clearing the body of antibiotic-resistant bacteria is particularly important when patients have repeat infections, as it’s likely these resistant bacteria are hiding out in the body between symptomatic episodes.
For example, urinary tract infections, some of the most common infections in the world, are often caused by resistant bacterial populations persisting in the body. Clearing the body of resistant bacteria would also be useful prior to surgery or chemotherapy, to prevent opportunistic infections while the body’s immune system is compromised.
Because of its targeted nature, the new drug combination is also predicted to be gentler on the body’s microbiome – the ecosystem of bacteria that naturally live within our bodies and can help fight infections.
New uses for old drugs
Given the lack of new drugs in the pipeline it’s essential to develop new ways of using the existing stock of effective medicines to function in new ways
Andrew Edwards
The team have applied for a patent for their drug and are working on expanding the concept to other antibiotics. Lead researcher Dr Andrew Edwards, from the MRC Centre for Molecular Bacteriology and Infection at Imperial, said: “Given the lack of new drugs in the pipeline it’s essential to develop new ways of using the existing stock of effective medicines to function in new ways, to reduce their damaging effects on our resident ‘good bacteria’ and to slow the rate at which bacteria become resistant to them.”
Dr Thomas Webb and Dr Dominic Marshall came up with the concept in 2013, when they were first-year medical students at Imperial. They worked with Professor Alan Armstrong in the Department of Chemistry and Dr Andrew Edwards in the MRC Centre for Molecular Bacteriology and Infection at Imperial to develop the idea, funded by an Imperial Confidence in Concept award. After an initial prototype was successful, Dr Lindsay Evans, an EPSRC-funded EMBRACE fellow picked up the baton and worked with the research team to secure funding from the Wellcome Trust to develop the idea further, along with Dr Jim Spencer from the University of Bristol.
Source: Imperial College London
15.05.2019