Article • Therapeutic progress
Cancer: riding the wave of innovation
In haematology and medical oncology, there is always something new. However, the increasing stratification of cancer therapies presents an enormous challenge for clinical research.
Report: Michael Krassnitzer
Tumour cells – those altered genetically by mutation and thus ought to be recognised by the immune system and destroyed – manage to apply diverse molecular tricks to avoid attack by the immune system. Thus, they are invisible to the T-cells that wander through the organism hunting for foreign and modified substances. At the end of August, an innovative therapy CAR-T was approved for use in Europe. This treatment aims to ensure that T-cells identify tumours and can recognise them as a threat to the body.
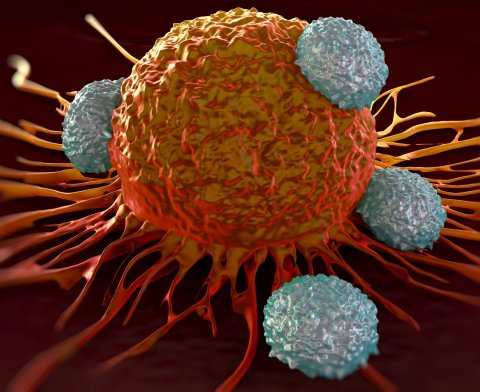
To this end, white blood cells are extracted from the patient and T-cells are derived from these in the laboratory. An inactive virus is inserted into them. The DNA in the T-cells adopts the virus’ heritage, expanded with a special gene. This gene ensures that the T-cells carry a certain protein in their shell, the chimeric antigen receptor against CD19. The receptor can recognise cancer cells in a patient and bond with them accurately. Chemotherapy is then used to kill as many of the patient’s T-cells as possible and the genetically modified CAR-T-cells are introduced by an infusion into the patient’s bloodstream. These cells multiply in the body and now actively fight tumour cells.
T-cell engineering was one of the focal points of this year’s meeting of the Austrian, German and Swiss specialist societies for haematology and medical oncology (DGHO, OeGHO, SGMO und SGH+SSH), held in Vienna this September. The CAR-T therapy is an expansion of the therapeutic portfolio by addition of a new principle for treatment of patients with malignant haematological illnesses, where initial studies show it to be in part highly effective. ‘In clinical studies, we reach response rates from 60 to 90% with acute leukaemia and 30 - 50% for non-Hodgkin lymphomas in advanced stages of illness. That is far more than with other therapies for these patients, who are very difficult to treat,’ explained Dr Hildegard Greinix, who heads the clinical department at the LKH – University Hospital Graz, and also serves as congress president of the annual meeting.
CAR-T-cells are just one example of the enormous therapeutic progress that has been reported, particularly in immunology-based anti-tumour therapies in recent years. The innovation wave rolls on with targeted cancer therapies oriented toward each tumour’s individual genetic properties.
This implies that, as a rule, clinical research today can no longer be conducted in one centre or one country, but networked worldwide in numerous centres
Andreas Petzer
Altogether the European Medicines Agency – EMA already approved 14 new medicines for haematology and medical oncology in 2016; 19 were approved in 2017. ‘That’s significantly more than in other fields,’ observed Dr Andreas Petzer, head of the internal oncology, haematology and gastroenterology department at the Ordensklinikum Linz, Sisters of Charity/Elisabethan Sisters, and president of the Austrian Society for Haematology and Medical Oncology (OeGHO).
Progress in basic research and, ultimately, increasingly stratified resp. personalised treatment of blood diseases and resulting solid tumours are a growing challenge. A newly developed medicine can no longer be applied globally for all patients of a certain entity. Instead they can now only be used for patients with a carcinoma exhibiting a very specific mutation. As a rule, this patient group is only a small sub-group of all patients. ‘This means that only a very specific segment of the patient pool is suitable for testing a special medicine in clinical studies and hence comparison with the past contribution of patients per clinical test naturally will be significantly reduced,’ Petzer explained. ‘This implies that, as a rule, clinical research today can no longer be conducted in one centre or one country, but networked worldwide in numerous centres.’
This means that in countries with small and medium-sized hospital structures – such as in Austria – careful consideration must be given as to which clinic or oncology centre applies for which study in order to remain interesting in the international competition as a partner for the pharmaceutical industry. If the agreed number of patients is not maintained, then, as a centre – and when this affects several centres, even as a country – one is quickly out of the race for future studies,’ the Austrian cancer specialist warns , ‘Therefore coordination of the clinical studies is necessary.’
Recommended article

Article • Chronic peripheral inflammation and schizophrenia
The network approach to mental illness research
As European health services are pressured to provide the best possible care for best possible value, some medical fields are now very much the poor relation; this is particularly true for mental health. Mental illnesses represent a great health burden and cause huge financial and societal pressure in terms of direct and indirect costs from repeated hospitalisation and treatment failures, while…
To remain competitive, despite increasingly differentiated therapeutic concepts, and to assure patients early access to innovative cancer medicines, the managing chair of the DGHO, Professor Michael Hallek, Director of Clinic One for Internal Medicine at the University Hospital Cologne, argues for a stronger integration of clinical research and patient care research in the sense of ‘knowledge-generating care’, where the data on therapy response, long-term outcome and undesired effects are assessed immediately. ‘With this approach, every treatment is ultimately and simultaneously clinical research too,’ Hallek explains. One of the prerequisites for such a change in thinking is comprehensive patient registers that can be analysed in real time, or at least very quickly. ‘That can only succeed with determined cross-institutional, intelligent and standardised digitisation of care data,’ the German expert emphasised. Such networks and registers are already being established in the United States and, according to Hallek, also ought to be implemented rapidly in Europe.
06.02.2019