Image source: Unsplash/ThisisEngineering RAEng
News • Neoadjuvant chemotherapy
Breast cancer: New test predicts therapy success
In a collaboration with the Faculty of Statistics at TU Dortmund and the University Medical Center in Mainz, a research team at the Leibniz Research Centre for Working Environment and Human Factors in Dortmund (IfADo) has developed a test that can be used to predict the success of therapy for breast cancer.
Breast cancer is one of the most common tumour diseases worldwide. One in eight women will develop it in the course of their lives. Even though the success of treatment has improved in recent decades, one in 39 women still dies of breast cancer. This makes breast cancer the second most common cause of death among tumour diseases in women, only lung cancer is more frequent. Breast cancer is often treated with so-called neoadjuvant chemotherapy. This serves to first reduce the size of a locally advanced tumour so that it can be operated on more easily. The chance of a cure depends very much on how well the patients respond to the neoadjuvant therapy. With previous methods, it was only possible to predict the success of the therapy inaccurately.
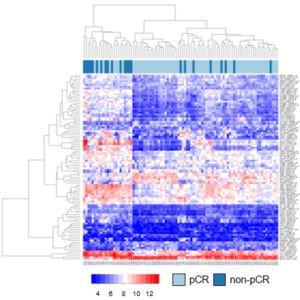
Foto: IfADo
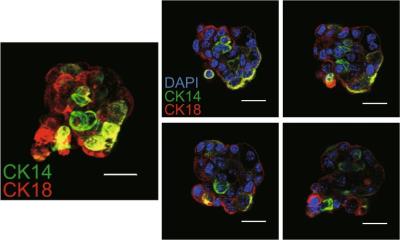
In cooperation with the Department of Obstetrics and Gynecology at University Medical Center in Mainz and the Faculty of Statistics at TU Dortmund, an IfADo research team in the Department of Toxicology has succeeded in developing a test that can be used to predict the response of the therapy (neoadjuvant chemotherapy). The tests used biopsy tissue that is routinely taken from breast cancer, so that all genes expressed in the tumour tissue are included in the procedure. The predictive test was developed in 114 patients and then validated in 619 independent patients. The method was recently published in the journal Clinical Cancer Research.
The negative predictive value of the test is 0.986. This means that at least 98 out of 100 women with a negative test result will actually have a tumour that responds poorly to the therapy. Such a prediction is important when new drugs are being developed. If it is possible to predict the failure of a particular type of therapy, alternatives can be considered for individual patients.
Source: IfADo – Leibniz Research Centre for Working Environment and Human Factors
06.07.2021