News • Innovative approach
New “Swiss Army Knife” nanovaccine to battle tumors
Vaccine stimulates multi-pronged immune attack, inhibits tumor-induced immune suppression
Scientists are using their increasing knowledge of the complex interaction between cancer and the immune system to engineer increasingly potent anti-cancer vaccines. Now researchers at the National Institute of Biomedical Imaging and Bioengineering (NIBIB) have developed a synergistic nanovaccine packing DNA and RNA sequences that modulate the immune response, along with anti-tumor antigens, into one small nanoparticle. The nanovaccine produced an immune response that specifically killed tumor tissue, while simultaneously inhibiting tumor-induced immune suppression. Together this blocked lung tumor growth in a mouse model of metastatic colon cancer.
The molecular dance between cancer and the immune system is a complex one and scientists continue to identify the specific molecular pathways that rev up or tamp down the immune system. Biomedical engineers are using this knowledge to create nanoparticles that can carry different molecular agents that target these pathways. The goal is to simultaneously stimulate the immune system to specifically attack the tumor while also inhibiting the suppression of the immune system, which often occurs in cancer patients. The aim is to press on the gas pedal of the immune system while also releasing the emergency brake.
A key hurdle is to design a system to reproducibly and efficiently create a nanoparticle loaded with multiple agents that synergize to mount an enhanced immune attack on the tumor. Engineers at the NIBIB report the development and testing of such a nanovaccine in the November issue of Nature Communications.
Making all the parts fit
Shrinking the particle is a critical step for activating an immune response
Guizhi Zhu
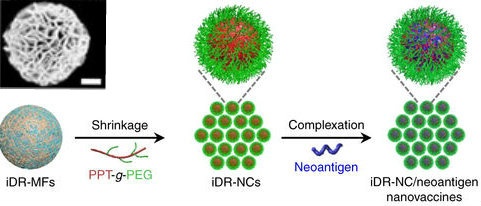
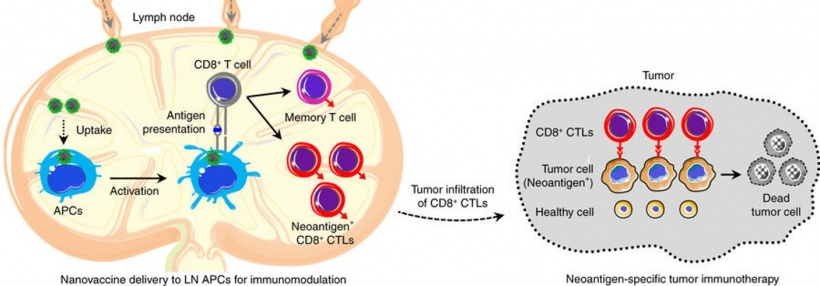
Guizhi Zhu, Ph.D., a post-doctoral fellow in the NIBIB Laboratory of Molecular Imaging and Nanomedicine (LOMIN) and lead author on the study, explains the challenge. “We are very excited about putting multiple cooperating molecules that have anti-cancer activity into one nanovaccine to increase effectiveness. However, the bioengineering challenge is fitting everything in to a small particle and designing a way to maintain its structural integrity and biological activity.” Zhu and his colleagues have created what they call a “self-assembling, intertwining DNA-RNA nanocapsule loaded with tumor neoantigens.” They describe it as a synergistic vaccine because the components work together to stimulate and enhance an immune attack against a tumor.
The DNA component of the vaccine is known to stimulate immune cells to work with partner immune cells for antitumor activation. The tumor neoantigens are pieces of proteins that are only present in the tumor; so, when the DNA attracts the immune cells, the immune cells interact with the tumor neoantigens and mount an expanded and specific immune response against the tumor. The RNA is the component that inhibits suppression of the immune system. The engineered RNA binds to and degrades the tumor’s mRNA that makes a protein called STAT3. Thus, the bound mRNA is blocked from making STAT3, which may suppress the immune system. The result is an enhanced immune response that is specific to the tumor and does not harm healthy tissues.
In addition to engineering a system where the DNA, RNA and tumor neoantigens self-assemble into a stable nanoparticle, an important final step in the process is shrinking the particle. Zhu explains: “Shrinking the particle is a critical step for activating an immune response. This is because a very small nanoparticle can more readily move through the lymphatic vessels to reach the parts of the immune system such as lymph nodes. A process that is essential for immune activation.” The method for shrinking also had to be engineered. This was achieved by coating the particle with a positively charged polypeptide that interacts with the negatively charged DNA and RNA components to condense it to one-tenth of its original size.
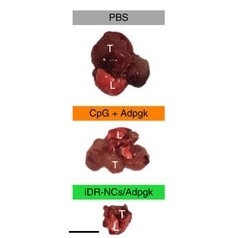
Testing the nanovaccine
To create a model of metastatic colon cancer, the researchers injected human colon cancer cells into the circulation of mice. The cells infiltrate different organs and grow as metastatic colon cancer. One of the prime sites of metastasis is the lung. The nanovaccine was injected under the skin of the mice 10, 16, and 22 days after the colon cancer cells were injected. To compare to the nanovaccine, two control groups of mice were analyzed; one group was injected with just the DNA and the neoantigen in solution but not formed into a nanovaccine particle, and the second control group was injected with an inert buffer solution.
At 40 days into the experiment, lung tumors from the nanovaccine-treated and the control groups were assessed by PET-CT imaging, and then removed and weighed. In mice treated with the nanovaccine, tumors were consistently one tenth the size of the tumors that were found in mice in both control groups. Further testing revealed that mice receiving the nanovaccine had a significant increase in circulating cytotoxic T lymphocytes (CTLs) that specifically targeted the neoantigen on the colon cancer cells. CTLs are cells that attack and kill virus-infected cells and those damaged in other ways, such as cancerous cells.
An important aspect of the nanovaccine approach is that it mounts an anti-tumor immune response that circulates through the system, and therefore is particularly valuable for finding and inhibiting metastatic tumors growing throughout the body. The researchers view their nanovaccine as an important part of eventual therapies combining immunotherapy with other cancer killing approaches.
Source: National Institute of Biomedical Imaging and Bioengineering
30.11.2017