Cancer diagnostics II
New nanoparticle could enhance MRI scanning
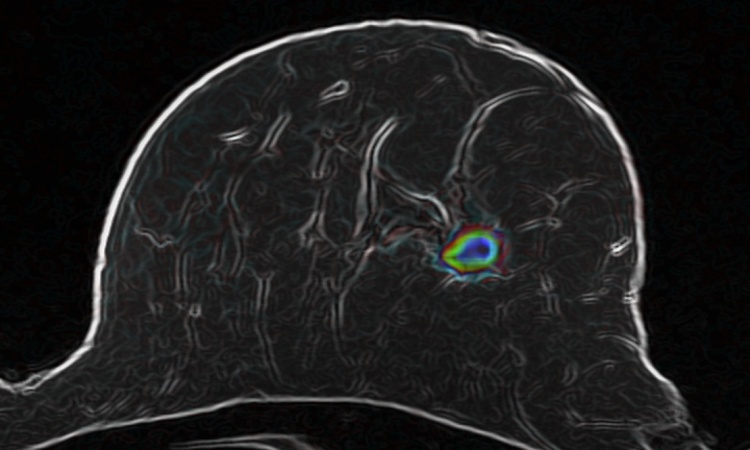
Scientists in the UK have designed a new self-assembling nanoparticle that targets tumours and could lead to quicker diagnosis of cancer. Researchers at Imperial College London report that a new self-assembling nanoparticle can adhere to cancer cells, thus making them visible in MRI scans and possibly eliminate the need for invasive tissue biopsies.
Report: Mark Nicholls
The nanoparticle increases the sensitivity and improves the efficacy of MRI scanning by ‘specifically seeking out receptors found in cancerous cells’ – a breakthrough the research team suggests has the potential to alter anatomic pathology’s role in diagnosing cancer and improve doctor’s ability to detect cancerous cells at much earlier stages of development.
Work is now under way to enhance the effectiveness of the newly designed nanoparticle as a tool to improve the sensitivity of MRI scanning, with a goal to test the design in a human trial within three to five years. Under the process, the nanoparticle is coated with a protein that looks for specific signals given off by tumours, and when a tumour is found it begins to interact with the cancerous cells. This interaction strips off the protein coating, causing the nanoparticle to self-assemble into a much larger particle so that it is more visible on the scan.
For the study, the ICL team used cancer cells and mouse models to compare the effects of the self-assembling nanoparticle in MRI scanning against commonly used imaging agents and found that the nanoparticle produced a more powerful signal and created a clearer MRI image of the tumour.
Professor Nicholas Long, at the Department of Chemistry at Imperial College London, said the results show real promise for better cancer diagnosis. ‘By improving the sensitivity of an MRI examination, our aim is to help doctors spot something that might be cancerous much more quickly,’ he explained. ‘This would enable patients to receive effective treatment sooner, which would hopefully improve survival rates from cancer.’
‘MRI scanners are found in nearly every hospital and are vital machines used every day to scan patients’ bodies and get to the bottom of what might be wrong; but we are aware that some doctors feel that, even though MRI scanners are effective at spotting large tumours, they are perhaps not as good at detecting smaller tumours in the early stages.’
The aim is to improve the design so that doctors can more easily spot a tumour and surgeons can then operate on it. Long: ‘We’re now trying to add an extra optical signal, so that the nanoparticle would light up with a luminescent probe once it had found its target, so combined with the better MRI signal this will make it even easier to identify tumours.’
The next research stage will endeavour to fine-tune the size of the final nanoparticle, to be even smaller but still produce an enhanced MRI image. However, researchers are aware that, if the nanoparticle is too small, the body will secrete it before imaging, but if too big it could be harmful. ‘Getting it just right is really important before moving to a human trial,’ added Dr Juan Gallo from the Department of Surgery and Cancer at ICL.
* Research funding came from Cancer Research UK, Engineering and Physical Sciences Research Council (EPSRC), the Medical Research Council (MRC) and the Department of Health.
PROFILE:
Professor Nicholas Long is the Sir Edward Frankland BP Chair in Inorganic Chemistry and Head of the Catalysis, Sustainability and Applied Inorganics Research Section at Imperial College London. The Long Group has expertise in applied synthetic inorganic and organometallic chemistry and his research interests focus on transition metal and lanthanide chemistry for the synthesis of functional molecules, homogeneous catalysis and, in recent years, probe design and novel methodologies for biomedical imaging. He has published c. 150 papers, several high impact review articles and the critically acclaimed textbook, Metallocenes. He received the 2006 RSC Prize in Organometallic Chemistry, was a Leverhulme Trust Research Fellow 2009/10 and, in 2011, became a Fellow of the Royal Society of Chemistry.
27.04.2015